A population based analysis of prognostic factors in advanced biliary tract cancer
Introduction
Biliary tract cancers (BTCs) are rare carcinomas that arise from the epithelial lining of the gallbladder and bile ducts. BTCs affect approximately 12,000 people in the U.S. annually (1). Complete surgical resection offers the only possibility for cure (a2, a3, a4). Among the minority of patients who undergo curative-intent resection, recurrence rates are high (2-4). Therefore, for the majority of BTC patients, systemic chemotherapy is the mainstay of their treatment plan.
Traditionally BTCs were divided into cancers of the extrahepatic ducts, the gallbladder, and the ampulla of Vater, while intrahepatic tumors used to be classified as primary liver cancers. More recently, bile duct cancers have been referred as cholangiocarcinomas, including cancers arising in the intrahepatic, perihilar, or distal biliary tree. Cancers of the gallbladder and ampulla of Vater, although part of the biliary drainage system, are currently considered as separate disease processes. However, these entities have traditionally been included together in clinical trials.
Among the BTCs, there are differences with respect to disease course. For instance, gallbladder cancers tend to recur systemically after curative-intent surgery, while hilar cholangiocarcinomas are more prone to have an isolated locoregional recurrence as the first site of failure (3). Moreover, it is believed that gallbladder carcinoma is a more aggressive disease than cholangiocarcinoma. A subgroup analysis of a large retrospective study of 104 advanced biliary tract carcinoma chemotherapy trials including 2,810 patients showed shorter overall survival (OS) for gallbladder carcinoma when compared with cholangiocarcinoma, despite the fact that gallbladder cancers had superior response rates (5).
Data regarding prognostic factors in advanced BTC remains scarce. The ABC-02 trial, the largest ever phase III trial in advanced BTC, randomized 410 patients to receive either gemcitabine alone or in combination with cisplatin and found a significant survival advantage for the latter (6). Since then, cisplatin and gemcitabine has become the standard of care for advanced BTC. However, prognostic factors among patients with advanced BTC treated in the routine clinical practice are non-existent. The aim of this study was to review our institutional experience in advanced BTC treated with first-line cisplatin and gemcitabine as well as to evaluate potential prognostic factors for survival.
Methods
A total of 106 patients with advanced BTC who initiated palliative chemotherapy with cisplatin and gemcitabine from 2009 to 2012 were identified using the British Columbia (BC) pharmacy database. BC is a Canadian province with a population of approximately 4.4 million, and two thirds of all systemic cancer treatments are delivered in five major cancer centers and their satellite clinics. Eligibility for the funded use of cisplatin and gemcitabine in BC for advanced BTC is typically restricted to patients with Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2, adequate marrow reserve (ANC greater than or equal to 1.5×109/L and platelets greater than 100×109/L), and adequate renal function (creatinine clearance above 60 mL/min).
Baseline demographics, tumor characteristics, treatment details and outcomes were abstracted to an anonymized database and analyzed. Primary tumor sites were defined according to cross-sectional imaging studies, surgery reports, discharge summaries or medical notes. Tumors were classified into gallbladder carcinoma, intra-hepatic cholangiocarcinoma, extra-hepatic cholangiocarcinoma, ampullary carcinoma or unknown primary tumor. All imaging performed prior to initiation of first-line chemotherapy were reviewed to investigate for sites of metastatic disease. Patients were classified as “locoregional only” if they had unresectable primary tumors, local disease recurrence (hepatic resection margin, bilioenteric anastomosis, or porta hepatic) and/or evidence of retroperitoneal lymphadenopathy. Those with metastatic disease limited to the liver were classified into “liver only”. Patients with evidence of other distant metastasis (with or without liver involvement) were classified as “other”. Disease progression was determined by review of sequential CT scans.
Statistical analysis
Statistical analysis was performed using SPSS version 14.0 for Windows® (SPSS, Chicago, IL, USA). OS was calculated in months from the time of chemotherapy initiation to date of death or censored at last follow-up. Kaplan-Meier curves for OS were generated. The log-rank test was used to assess statistical differences among variables and P values <0.05 were considered statistically significant.
Multivariable survival analyses were performed using Cox proportional hazards regression models to determine the association between local primary sites and OS outcomes after adjustment for potential confounders. Variables in the model included factors significant on univariate analysis. Hazard ratios (HR) and 95% confidence intervals were calculated to estimate risk of death.
Results
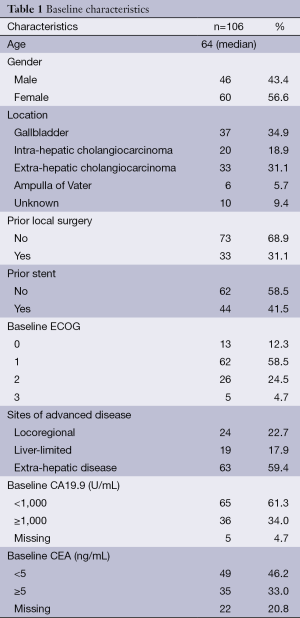
Median follow-up time was 29.4 months. In our cohort, 106 patients with advanced BTC began palliative chemotherapy with cisplatin and gemcitabine. Their characteristics are summarized in Table 1. Median age was 64 years (range 43-88). There were 60 (56.6%) females and the majority of patients had a baseline ECOG PS of 1 (n=62, 58.5%), followed by 2 (n=26, 24.5%). Only 13 patients were ECOG PS 0 (12.3%) and 5 were ECOG 3 (4.7%). The majority had gallbladder carcinoma (n=37, 34.9%), followed by extra-hepatic cholangiocarcinoma (n=33, 31.1%), intra-hepatic cholangiocarcinoma (n=20, 18.9%) and ampullary carcinoma (n=6, 5.7%). Primary location was unknown in 10 patients (9.4%).
Full table
Regarding sites of metastasis, 24 patients (22.7%) had locoregional disease, 19 (17.9%) had liver-limited metastasis and 63 (59.4%) had other sites of metastasis. Thirty-three patients (31.1%) had previously undergone curative-intent surgery and 44 (41.5%) had a biliary stent placed prior to embarking on chemotherapy. Median baseline CA19-9 and CEA were 300 U/mL and 3.2 ng/mL, respectively. Thirty-nine patients (36.8%) received second-line chemotherapy, with single-agent 5-fluorouracil being the most used drug (n=12).
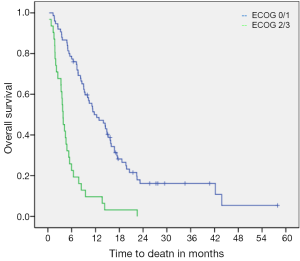
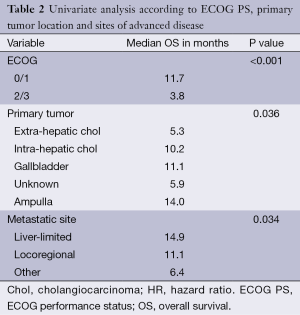
Median OS from initiation of chemotherapy to death was 8.5 months (95% CI: 6.5-10.5). On univariate analysis, ECOG PS 2/3 was significantly associated with worse OS (median OS was 11.7 months for ECOG 0/1 versus only 3.8 months for ECOG 2/3, P<0.001) (Figure 1). Primary tumor locations were also found to be statistically prognostic for OS (P=0.036) (Table 2). Patients with gallbladder carcinoma had a median OS of 11.1 months. Those with intra-hepatic and extra-hepatic cholangiocarcinoma had median OS of 10.2 and 5.3 months, respectively. Patients with ampullary carcinoma had a median OS of 14.0 months while those with unknown primary tumors had a median OS of only 5.9 months.
Full table
Sites of advanced disease were also found to be prognostic of OS (P=0.034) (Table 2). Patients with locoregional disease, liver-limited metastasis and other metastatic sites had median OS of 11.1, 14.9 and 6.4 months, respectively. Gender and age (<65 vs. ≥65) were not prognostic for OS on univariate analysis (P=0.502 and 0.292, respectively). Biliary stent prior to chemotherapy initiation and baseline CA19-9 (<1,000 vs. ≥1,000 U/mL) also had no impact on OS (P=0.603 and 0.372). Baseline hemoglobin (<120 vs. ≥120 g/L) and neutrophils (<5 vs. ≥5×109/L) were also not prognostic of OS (P=0.371 and 0.251, respectively). There was a trend towards worse OS for patients with baseline CEA ≥5 ng/mL (5.2 vs. 12.9 months, P=0.05).
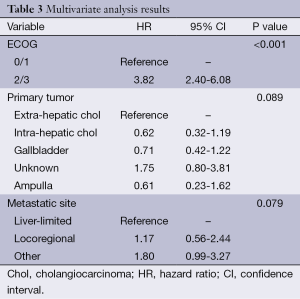
On multivariate analysis, ECOG PS 2/3 was significantly associated with worse OS (HR 3.82, P<0.001). There was a trend towards worse OS for patients with other sites of metastatic disease as compared to liver-limited metastasis (HR 1.80, P=0.079). In addition, there was also a trend towards prognostic significance for primary tumor location on OS (P=0.089) in which patients with extra-hepatic cholangiocarcinoma and unknown primaries seemed to have worse outcomes. Table 3 shows the results of the multivariate analysis.
Full table
Discussion
To the best of our knowledge, this is the first study to investigate predictors of survival in BTC among patients who underwent palliative chemotherapy with cisplatin and gemcitabine outside of a clinical trial. Our study demonstrates that ECOG PS is the main prognostic factor for OS. Although not reaching statistical significance on multivariate analysis, different primary sites tended to have distinct outcomes. Extra-hepatic cholangiocarcinomas and unknown biliary location had the worse OS (5.3 and 5.9 months, respectively), while gallbladder and ampullary carcinoma were associated with better median OS (11.1 and 14.0 months, respectively). This is in contrast to a pooled analysis of 104 studies which showed worse OS among gallbladder carcinoma (5).
The superiority of gemcitabine plus cisplatin over gemcitabine alone was shown in the multicenter ABC-02 trial (6). The trial data was then used to develop a risk model for BTC patients addressing both potential prognostic and predictive factors (7). The baseline demographic and clinical characteristics, including tumor markers, of the patients were tested for their association with OS. Their multivariate analysis demonstrated that the site of the tumor within the biliary tract did not affect survival. Patients with tumors in the gallbladder had a median survival of 9.6 months compared to 9.1 months for tumors in other parts of the biliary tract (P=0.855). Our study also showed that gallbladder carcinoma is not associated with worse OS, as previously thought. It remains unclear why patients with extra-hepatic cholangiocarcinoma had poor outcomes in our analysis as compared to prior studies (7,8). The ABC-02 trial did not include patients with metastatic cholangiocarcinoma of unknown primary and our study suggests that those patients have short OS when treated with cisplatin and gemcitabine.
A Korean series of 213 patients with advanced BTC receiving first-line chemotherapy showed that metastatic disease, intrahepatic cholangiocarcinoma, the presence of liver metastases, ECOG PS and elevated level of serum alkaline phosphatase were significant predictors of OS (8). Using these five variables, the authors developed a prognostic index to stratify patients into low-risk, intermediate-risk, and high-risk groups with different median (11.5, 7.3, and 3.6 months, respectively) and 1-year survivals (48, 26, and 4 percent, respectively). Our study did not show inferior outcomes for patients with liver involvement, or for patients with intra-hepatic cholangiocarcinoma. In addition, the Korean results cannot be applied for patients who receive cisplatin and gemcitabine as first-line chemotherapy given the fact patients were treated with capecitabine and cisplatin (n=42), gemcitabine and capecitabine (n=38) or S-1 monotherapy (n=133) in their study (8).
Similar to our findings, patients with ECOG 2 enrolled in the ABC-02 trial did worse than those with ECOG 0/1 (HR 2.19, P<0.001), suggesting that PS is an important driver of OS (7). It is possible that the lesser proportion of patients with ECOG 2 (13.2%) in the experimental arm of the ABC-02 trial may have contributed to a longer OS when compared to our cohort, where 29.2% of the patients had ECOG ≥2. Metastatic disease was also associated with a worse OS than locally advanced disease in the ABC-02 trial (HR 1.32, P=0.031). We also showed worse OS for patients with distant metastatic disease, but not for those with liver involvement only. Neutrophilia and anemia at baseline were both associated with significantly poorer survival in the trial, which was not confirmed in our population-based study. Likewise our study, CA19.9 and CEA levels were not correlated with OS (6).
There are several limitations to the current study. First, the retrospective, non-randomized nature of this review relies on accuracy of written records and information captured by them. Second, we were unable to evaluate overall response rates, toxicity and discontinuation rates, which would otherwise strengthen our results. Moreover, our relatively small cohort size limits the power of the statistical analyses. Nonetheless, this is the largest population-based analysis to report prognostic factors in BTC patients who embark on cisplatin and gemcitabine.
Conclusions
Our study shows that among BTCs treated with gemcitabine and cisplatin, the main factor associated with survival is baseline performance status. There were non-significant differences in outcomes by disease site, and location of metastasis, and further work to confirm the prognostic implications of these factors is warranted.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66. [PubMed]
- Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463-73; discussion 473-5. [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. [PubMed]
- Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007;31:1256-63. [PubMed]
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007;96:896-902. [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [PubMed]
- Wasan HS, Walle JW, Palmer DH, et al. Predictors of survival in patients with advanced biliary tract cancer: Results from the UK ABC-02 randomized phase III trial. 2010 Gastrointestinal Cancers Symposium, Abstract 199.
- Park I, Lee JL, Ryu MH, et al. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer 2009;115:4148-55. [PubMed]


