Incidence and risk factors for post-operative mortality, hospitalization, and readmission rates following pancreatic cancer resection
Introduction
Pancreatic cancer is a highly lethal malignancy affecting over 50,000 patients each year in the United States (1). To date, surgery remains the only potentially curative option for pancreatic cancer (2). However, due to a high proportion of patients presenting at advanced stages, only 15–20% of patients ultimately end up being eligible for surgery (3). The most common oncologic surgery is pancreaticoduodenectomy, which is a technically complicated, high-risk procedure. The best outcomes following the procedure have been reported at high-volume centers, with mortality rates in the 3–5% range (4,5). Generally speaking, guidelines define resectability along a continuum based on involvement of nearby normal structures and vasculature, as well as presence or absence of metastatic disease (6,7). In addition, patient comorbidities, physician bias, and other socioeconomic factors play a role in whether or not surgical resection is offered or attempted, as well as outcomes thereof (8). With that in mind, we sought to use the National Cancer Database (NCDB) to examine 30-day and 90-day post-operative mortality following contemporary pancreatic cancer surgery across the United States, as well as evaluate predictors for worse outcomes.
Methods
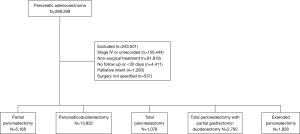
The NCDB is overseen by the American College of Surgeons and the Commission on Cancer and encompasses an estimated 70% of annual newly diagnosed cancer cases in the United States. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. Given its retrospective nature and de-identified dataset, this study was exempt from institutional review board approval. We queried the NCDB from 2004–2015 for patients with stage 1–3 adenocarcinoma of the pancreas treated surgically with at least 30 days of follow up. Figure 1 is a CONSORT diagram outlining all inclusion and exclusion criteria.
Surgery is coded in the NCDB based on degree of pancreatic removal and gastroduodenal resection. These were grouped into categories of pancreaticoduodenectomy, partial pancreatectomy (e.g., selective removal of the pancreatic body or tail with or without other organs such as the spleen), total pancreatectomy (removal of the entire pancreas) with or without subtotal resection of the duodenum and/or stomach, and extended pancreatectomy. Of note, the definition of extended pancreatectomy is more difficult to ascertain because it has utilized various definitions in relation to resection of adjacent organs (e.g., colon) with or without arterial and/or venous structures (9). Although a more unified definition was proposed in mid-2014 (10), it is unlikely that most patients herein were defined by the unified terminology because a minority of patients in the NCDB were treated in 2014–2015.
The NCDB contains various patient-related and socioeconomic factors ranging from race, median household income, type of insurance, gender, age, education level, distance from treatment facility, and comorbidity score. Race was simplified into 3 categories: Caucasian, African American, or other. The Charlson/Deyo comorbidity index (a widely used scale) was recorded and quantified the degree of comorbidities in the patient population (11). Age was broken up into 5 groups; <50, 50–59, 60–69, 70–79, and ≥80. Socioeconomic data in the patients’ residence census tract were divided into quartiles based upon the percentage of persons with less than a high school education and median household income based on zip code of residence. Facility type was grouped according to the Commission on Cancer accreditation category (community cancer center, comprehensive community cancer center, and academic/research program). Locations were described based on data provided by the US Department of Agriculture Economic Research Service. Insurance status is documented in the NCDB as it appears on the admission page.
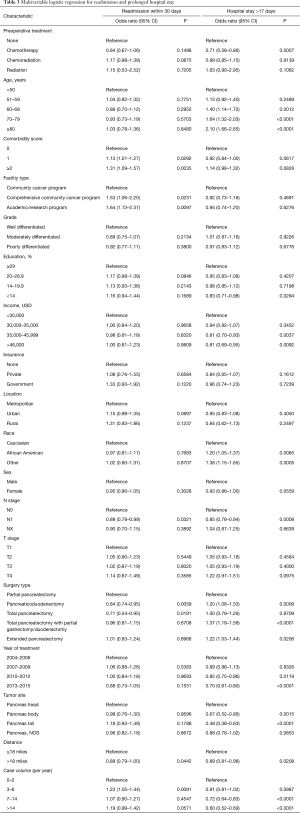
Statistical methodology for this study mirrored analogous impactful publications in other neoplasms (12). Data were analyzed using Medcalc Version 18 (Ostend, Belgium). Summary statistics are presented for discrete variables. Sociodemographic, treatment, and tumor characteristics were first tabulated. Multivariable logistic regression was done to identify predictors of 30-day mortality, 90-day mortality, readmission within 30 days of surgery, and prolonged hospital stay (defined as >17 days based on receiver operator curve analysis). Number of pancreatic surgeries per year was calculated by tabluting the number of times, a facility ID was listed in the dataset, and then dividing that sum by the number of years. That value was then split into quartiles to create 4 different groups of surgical volume. Adjusted hazard ratios and 95% confidence intervals are reported, using an alpha level of 0.05 to indicate statistical significance.
Results
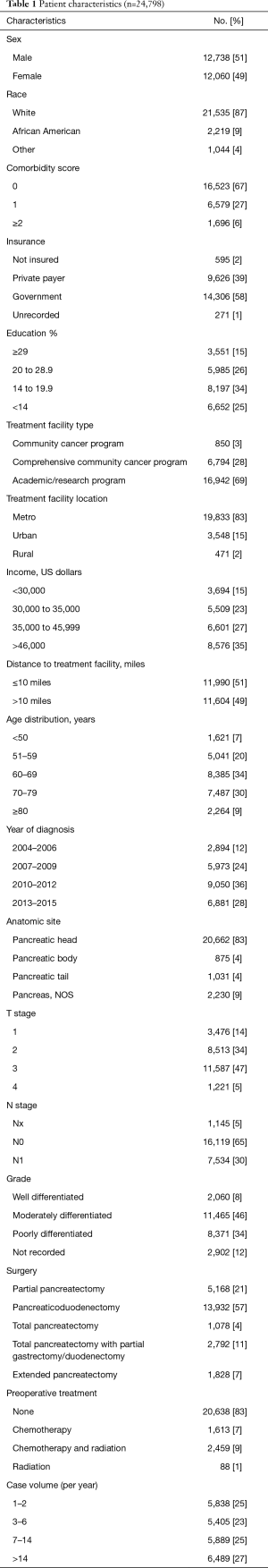
Using the above eligibility criteria, we identified 24,798 patients with pancreatic adenocarcinoma treated surgically. Table 1 outlines baseline patient characteristics for the entire cohort. Briefly, the majority were lesions of the pancreatic head (83%), stage T3 (47%), N0 (65%), and treated with pancreaticoduodenectomy (57%). The median age across the cohort was 66 [40–90]. Eighty-three percent of patients were treated without any neoadjuvant therapy; 7% and 9% received neoadjuvant chemotherapy or chemoradiation, respectively. The median radiation dose in that subset was 50 Gy (interquartile range, 45–50.4 Gy) in 28 fractions (interquartile range, 25–28 fractions). The median time from diagnosis to surgery was 21 days (interquartile range, 6–47 days). Median follow up for the entire cohort was 20 months (range, 1–155).
Full table
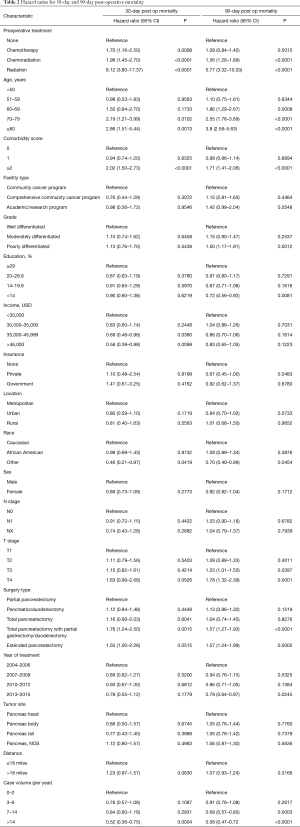
The global rate of 30-day and 90-day post-operative mortality was 1.7% and 5%, respectively. On multivariable analysis, predictors of 30-day mortality included preoperative therapy, increasing age, higher comorbidity score, lower income, lower case volume, and type of surgery (total pancreatectomy with duodenal/gastric resection, or extended pancreatectomy) (Table 2). Ninety day mortality was associated with preoperative therapy, increasing age, higher comorbidity score, higher T stage, lower case volume, and type of surgery (total pancreatectomy with duodenal/gastric resection, or extended pancreatectomy) (Table 2).
Full table
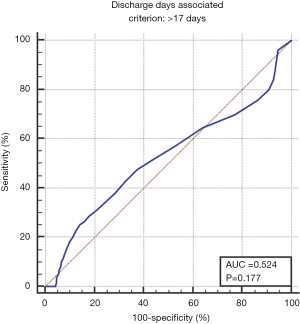
The median hospital stay was 9 days (interquartile range, 7–13 days). Receiver operating curve analysis identified 17 days as indicative of higher mortality, and was thus used to define a prolonged hospital course (Figure S1). The global rate of patients experiencing hospitalization >17 days was 14% for the entire cohort. Multivariable analysis identified the following to associate with prolonged hospital stay: increasing age, lower education, lower income, non-Caucasian race, lower case volume, and more extensive surgery (i.e., techniques involving bowel removal) (Table 3). We also looked at readmission rates, and across all 24,798 patients 32% ended up with a readmission. On multivariable analysis, similar variables to those for prolonged hospital stay predicted for 30-day readmission (Table 3).
Full table
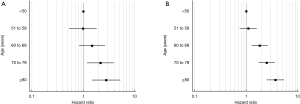
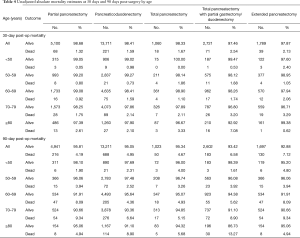
We also calculated absolute rates of 30- and 90-day mortality for each age group by surgical approach (Table 4). Post-operative mortality increased across all age groups (Figure 2) and also by extent of surgery [with a dip for the oldest group or patients and extended pancreatectomy (likely owing to small sample sizes)] (Figure 3). Age ≥70 was most associated with 30-day mortality, whereas age ≥60 was most associated with 90-day mortality and prolonged hospital stay. Mortality rates peaked at 30 and 90 days for the oldest group of patients treated with a total pancreatectomy with partial gastrectomy (7.08% and 13.27%, respectively).
Full table
Discussion
This study of a large, contemporary national database illustrates incidences of, and risk factors for, post-procedural mortality, along with those for prolonged hospital stay and 30-day readmission. Although resection is the only potentially curative option for pancreatic cancer, quantitation of incidences and risk factors for these outcomes is essential for judicious patient selection and shared decision-making between providers and patients. Data presented from the current analysis shows an acceptably low 30-day and 90-day mortality risk, mirroring existing studies (10-13). As expected, the risk of mortality increased with advancing age, in some cases eclipsing 10%. However, this must also be contextualized with the case volume and extent of surgery, which also were powerful predictors of postoperative outcomes. Other interesting findings include the potential protective nature of “other race” in this series, although we must admit that represents a heterogeneous population and was only 5% of our cohort allowing for possible bias. Additionally, older year of diagnosis/treatment and anatomic site predicted for prolonged hospital stay, likely showing an increase in surgical expertise and technology over time, as well as highlighting the increased difficulty of surgery on non-head lesions.
The increased mortality with neoadjuvant therapy may be from other disease features not captured in the NCDB, as opposed to a treatment-related detriment. Systematic analyses of prospective data have failed to numerically associate neoadjuvant chemotherapy or chemoradiation with worse postoperative complications or mortality (13). However, chemotherapy utilization has historically been singlet or doublet therapy, whereas multi-agent chemotherapy is expected to rise in the near future (14). The use of stereotactic body radiation therapy in the neoadjuvant setting has also increased, which requires further longitudinal assessment of complications, especially as related to technical planning parameters (15).
There are also data from single institutions as well as national databases to appropriately consent patients regarding potential post-operative risks. A large series from Memorial Sloan Kettering examined outcomes in close to 600 patients treated surgically between 2001 and 2009. In that series the rate of high grade complications (≥ grade 3) was 22% and 90-day mortality was excellent at 3.7%. High grade complications were not associated with any detriment to survival (16). The group from University of North Carolina used the Nationwide Inpatient Sample database to examine outcomes (specifically in-hospital mortality) in close to 25,000 patients treated with pancreaticoduodenectomy between 1988 and 1995 (17). The in-hospital mortality rate using that data was higher, 11%, in urban teaching hospitals. Granted, the number of procedures per year in their dataset was only 2–3 per year for teaching hospitals (i.e., significantly lower than the data cited above using a cutoff of 25 cases/year to define high volume). Another series used data from Veterans Affairs (VA) hospital systems and compared outcomes to those patients treated in the private sector with pancreatectomy (18). In total, over 1,000 patients were included, with VA patients having worse performance status and comorbidities. In terms of 30-day mortality, the rate was 6.4% in the VA system compared to 3.8% in the private sector (P=0.0015). Lastly, a more limited analysis of the NCDB from 2007–2010 examined 30-day and 90-day mortality following pancreatectomy (19). That study included over 21,000 patients revealing an overall 30 day mortality rate of 3.7%, which doubled to 7.4% at 90 days. Predictors for increased mortality included older age, government insurance, lower income, lower hospital volume, and higher comorbidity score.
Surgical technique also has the potential to play a role in post-operative outcomes. A relatively recent, small, randomized study (32 patients in each arm) from India directly compared a laparoscopic approach to traditional open pancreaticoduodenectomy (20). There was less blood loss and shorter hospital stay with the minimally invasive approach, with similar complication rate. The group from Memorial Sloan Kettering performed an earlier meta-analysis of 6 studies with over 500 patients comparing laparoscopic and open approaches (21). The findings of that study were that minimally invasive pancreaticoduodenectomy was a feasible approach with likely less blood loss and shorter hospital stay, keeping in mind possible selection bias as all the included studies were retrospective in nature. In the recent past, a robotic approach has been utilized with increasing frequency as well, with proposed advantages being positioning, better visualization, and increased dexterity. A multi institutional analysis reviewed outcomes in over 1,000 patients, of which 211 were treated with a robotic pancreaticoduodenectomy (22). Within that cohort, operative times were longer, but blood loss and major complications were reduced. Length of stay, readmission, and 90 day mortality were not associated with operative approach. Likewise, operative approach was not a predictor of margin status or extent of nodal dissection. The NCDB (2010–2012 version) has also been utilized to compare open approaches to minimally invasive approaches for pancreaticoduodenectomy (23). That particular analysis showed shorter length of stay, more margin-negative resections, and quicker time to adjuvant systemic therapy using a minimally invasive approach. There was no difference in survival based on operative technique. We also considered looking into surgical technique (open/laparoscopic/robotic) for the present analysis, and if it played any role in outcomes, but that data was not captured until 2010 (and only sparingly so thereafter).
The limitations of the current study include its retrospective nature and inherent selection bias. It is important to note however that unlike the majority of NCDB studies, much of the selection bias is mitigated since overall survival is not the main endpoint. The NCDB does not code completely for lack of or presence of vascular involvement which affect surgical outcomes and technique for pancreatic cancer, but since T4 by definition denotes vascular involvement; this may be an adequate surrogate. There are also no data captured on specific chemotherapy agents or number of cycles delivered. More specific toxicity and morbidity data is also lacking in the NCDB, which is also important to consider in the post-operative setting. Lastly, nuances of procedures are also not captured by the NCDB (e.g., pylorus preservation techniques, portal vein reconstructions, etc.).
Conclusions
This study of a large, contemporary national database illustrates incidences of, and risk factors for, post-procedural mortality, along with those for prolonged hospital stay and 30-day readmission. Although resection is the only potentially curative option for pancreatic cancer, quantitation of incidences and risk factors for these outcomes is essential for judicious patient selection and shared decision-making between providers and patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017;265:185-91.
- Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer 1995;76:1671-7. [Crossref] [PubMed]
- Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg 2007;245:777-83. [Crossref] [PubMed]
- Fong Y, Gonen M, Rubin D, et al. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg 2005;242:540-4; discussion 544-7. [PubMed]
- Network NCC. Pancreatic Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed January 14, 2019.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173-80. [Crossref] [PubMed]
- Fortner JG, Kim DK, Cubilla A, et al. Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg 1977;186:42-50. [Crossref] [PubMed]
- Hartwig W, Vollmer CM, Fingerhut A, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 2014;156:1-14. [Crossref] [PubMed]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [Crossref] [PubMed]
- Stokes WA, Bronsert MR, Meguid RA, et al. Post-Treatment Mortality After Surgery and Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:642-51. [Crossref] [PubMed]
- Verma V, Li J, Lin C. Neoadjuvant Therapy for Pancreatic Cancer: Systematic Review of Postoperative Morbidity, Mortality, and Complications. Am J Clin Oncol 2016;39:302-13. [Crossref] [PubMed]
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:963-9. [Crossref] [PubMed]
- Elhammali A, Patel M, Weinberg B, et al. Late gastrointestinal tissue effects after hypofractionated radiation therapy of the pancreas. Radiat Oncol 2015;10:186. [Crossref] [PubMed]
- Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol 2016;113:188-93. [Crossref] [PubMed]
- Kotwall CA, Maxwell JG, Brinker CC, et al. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol 2002;9:847-54. [Crossref] [PubMed]
- Glasgow RE, Jackson HH, Neumayer L, et al. Pancreatic resection in Veterans Affairs and selected university medical centers: results of the patient safety in surgery study. J Am Coll Surg 2007;204:1252-60. [Crossref] [PubMed]
- Swanson RS, Pezzi CM, Mallin K, et al. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the national cancer data base. Ann Surg Oncol 2014;21:4059-67. [Crossref] [PubMed]
- Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg 2017;104:1443-50. [Crossref] [PubMed]
- Correa-Gallego C, Dinkelspiel HE, Sulimanoff I, et al. Minimally-invasive vs open pancreaticoduodenectomy: systematic review and meta-analysis. J Am Coll Surg 2014;218:129-39. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- Mirkin KA, Greenleaf EK, Hollenbeak CS, et al. Minimally invasive surgical approaches offer earlier time to adjuvant chemotherapy but not improved survival in resected pancreatic cancer. Surg Endosc 2018;32:2387-96. [Crossref] [PubMed]