Is metastasectomy a worthy option?—the role of surgery in metastatic colon cancer to liver and lungs
Introduction
In 2014, 139,992 patients received a new diagnosis of colorectal cancer (CRC) in the United States and an additional 140,250 are expected in 2018; moreover, CRC is one of the most common causes of cancer death in both women and men (1). Thanks to the improvement of CRC screening programs, it has been possible for physicians to detect tumors at earlier stages (2,3). However, approximately 21% of new CRC cases are diagnosed at stage IV, for which the 5-year overall survival (OS) is 13% (4).
In recent years, several studies have been conducted in order to assess and refine systemic regimens for advanced and metastatic colon cancer (CC) (5-7). There is consensus regarding the use of a multidisciplinary approach to treat stage II and III CC (8); however, the role of surgery and especially metastasectomy is still controversial for treating stage IV CC (with the exception of symptomatic/palliative treatment), and discrepancies exist between guidelines and surgical practice (9,10). Some authors have found that both primary tumor resection (PTR) and metastasectomy—in addition to systemic therapy—improve the 5-year OS for metastatic CC from 20% to 50% (11,12).
The aim of this study was to compare survival rate in patients diagnosed with CC metastatic to liver with or without lung metastases who received surgery versus those who did not; subsequently we aimed to evaluate whether performing metastasectomy in addition to PTR improves OS.
Methods
Patients
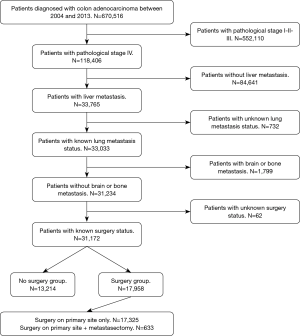
The National Cancer Data Base (NCDB) was retrospectively queried for patients diagnosed with colon adenocarcinoma between 2004 and 2013. Further inclusion criteria were pathological stage IV with liver metastasis and known status of lung metastasis. Lastly, only patients with known surgery status were included in the study. Patients with histology different from adenocarcinoma, carcinoma in situ, and more than 1 recorded malignancy were excluded from the analysis. Pathological stage I, II, III and IV with brain or bone metastasis were excluded as well. Figure 1 summarizes the adopted inclusion and exclusion criteria. As this study utilized a nationwide, de-identified database, it was deemed exempt from the Institutional Review Board.
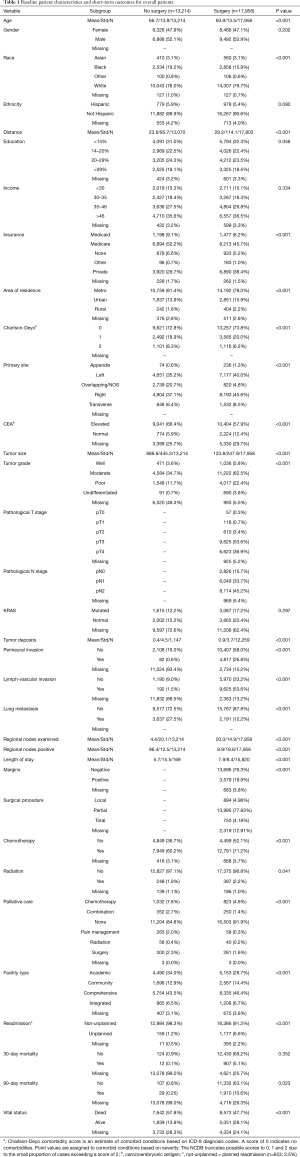
The following patient-specific characteristics were collected: age, gender, race, Hispanic ethnicity, education level, income, insurance status, area of residence and Charlson-Deyo score as a measure of comorbidity status. The tumor-related characteristics included primary site, preoperative carcinoembryonic antigen (CEA) levels, tumor grade, size, pathological T and N stage (7th edition AJCC staging), K-RAS status, presence of perineural and lymph-vascular invasion, and number of positive regional nodes. Treatment and outcome-related variables included margins status, number of regional nodes examined, administration of chemotherapy and radiotherapy, type of palliative care, facility type, length of stay, 30- and 90-day mortality, readmission, and vital status.
Statistical analysis
In our first analysis, patients were divided in 2 groups based on whether surgery was performed. Subsequently, patients who received surgery were divided in 2 groups based on whether metastasectomy was performed in addition to PTR. The associations of patient characteristics and short-term outcomes with groups were evaluated using the Kruskal-Wallis and Pearson Chi-square tests, as appropriate. OS was summarized by surgical group using standard Kaplan-Meier methods, where estimates of the median survival were obtained with 95% confidence intervals. The association between surgical group and OS was evaluated using the log-rank test. Statistically significant variables were subsequently included in a multivariate analysis and hazard ratio (HR) and its corresponding 95% CI were calculated for overall sample and stratified by group. All analyses were conducted in SAS v9.4 (Cary, NC, USA) at a significance level of 0.05.
Results
A total of 31,172 patients were included in the analysis after the application of the inclusion and exclusion criteria.
Analysis of the main cohort
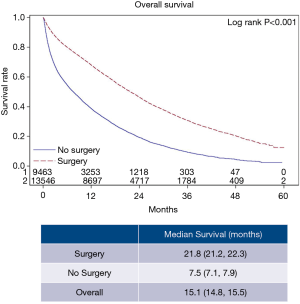
The overall patient characteristics and short-term outcomes are summarized in Table 1. Figure 2 shows the Kaplan-Meier curve for OS of both surgery and non-surgery groups. The median survival for all patients was 15.1 months, patients who underwent surgery survived longer compared to those who did not (P<0.001).
Full table
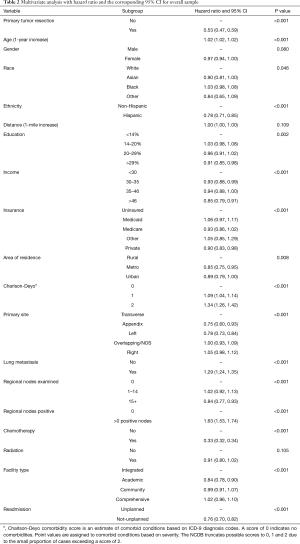
The results of the multivariate model used to calculate the HR for each variable are shown in Table 2. Patient characteristics significantly associated with better survival rate were Hispanic ethnicity, higher education and income, being covered by a private insurance, and residing in a metropolitan area. Conversely older patients who had a comorbidity score of 1 or more were associated with poorer outcomes. Undergoing surgery and/or receiving chemotherapy were associated with better survival outcomes, while radiation therapy was found not significant. When looking at tumor characteristics, better survival outcomes were observed in patients who had their primary tumor located in either the descending colon or the appendix, and who had more than 15 lymph nodes examined; on the contrary, patients with positive lymph nodes and lung metastasis had worse outcomes.
Full table
Analysis on surgical sub-cohort
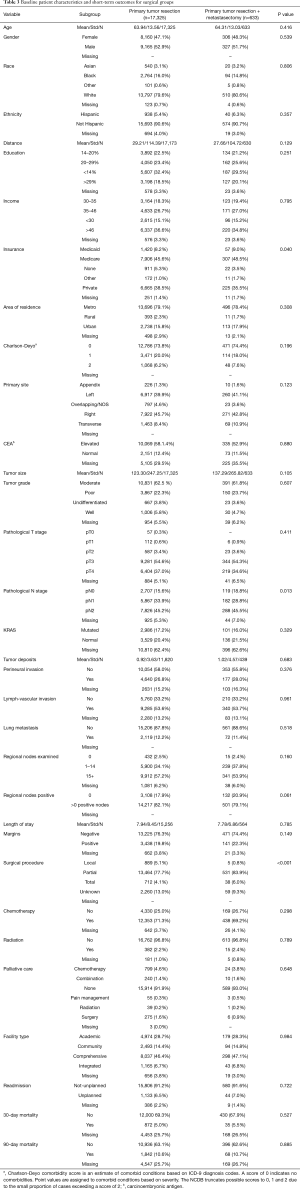
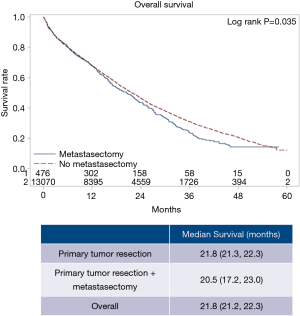
Specific characteristics and short-term outcomes for surgical patients are summarized in Table 3. Figure 3 shows the Kaplan-Meier curve for OS of both metastasectomy and non-metastasectomy groups. Patients who received PTR alone survived longer compared to those who underwent PTR and metastasectomy (P=0.035).
Full table
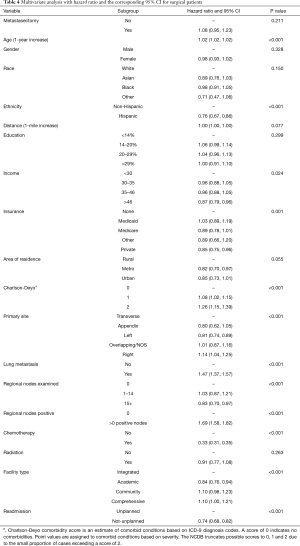
The results of the multivariate model used to calculate the HR for each variable are shown in Table 4. Patient characteristics associated with better survival rate were Hispanic ethnicity, higher income and being covered by a private insurance. Conversely, older patients who had a comorbidity score of 1 or more were associated with poorer outcomes. Tumor and treatment specific outcomes associated with better survival were the amount of regional nodes examined higher than 14, receiving chemotherapy, being treated at an academic center and not being readmitted unless planned. On the contrary, patients with regional nodes positive and lung metastasis were associated with poorer outcome. Furthermore, patients who had their primary tumor located in the descending colon were more likely to survive compared to those who had it in the ascending colon; the other sections of the colon were instead not significantly different in terms of survival. Ultimately receiving metastasectomy in addition to PTR was not associated with either lower or higher risk of death.
Full table
Discussion
In this retrospective analysis of the NCDB we report that PTR can be performed safely and is associated with improved survival—compared to systemic treatment only—for select patients with metastatic CC. The role of metastasectomy, however, is still not clear as no difference was found in our results.
Current clinical practice guidelines recommend that surgical resection of both primary tumor and metastases should be based on a multidisciplinary approach, but it is feasible and appropriate when tumor regression is obtained through systemic therapy. However, in the setting of unresectable stage IV disease, surgical approach is not indicated unless symptoms or complications related to the primary tumor occur (13). Multiple studies analyzed this aspect and reported that in select patients, PTR is associated with improved OS in association with systemic therapy (14,15), potentially even when performed as the first treatment step (16). However there is no consensus on the role of PTR as routine treatment for unresectable stage IV CC; several other studies reported increased morbidity and mortality and no improvement in OS for surgical patients (17,18). Furthermore there is a high risk that the improvement in OS reported by some studies was affected by selection bias and/or confounders because patients who are candidates for surgery are usually associated with less comorbidities, better performance status, less metastatic burden and, therefore, better chances to receive palliative systemic therapy (18).
Lung and/or liver metastasectomy has been reported beneficial for eligible patients who undergo a multidisciplinary evaluation aiming to define an individualized treatment strategy (19,20). In select patients, it is in fact associated with improved survival rate (21) and repeated metastasectomy has been demonstrated a valid option as well (22,23).
Our results show that PTR improves survival outcomes for patients with stage IV CC. Patients who underwent surgery had in fact 47% lower risk of death; their median survival was also considerably higher compared to patients who did not receive surgery.
When looking at patients who underwent surgery (either PTR alone or PTR with metastasectomy), no difference was found between the 2 groups regarding mortality. Conversely, median survival was statistically significant in favor of patients who did not receive metastasectomy. However, this finding might not have a relevant clinical significance; it would be expected, in fact, that patients undergoing PTR with metastasectomy have a higher mortality rate due to the more extensive treatment they receive and the related complications and side effects. It is possible that surgeons selected patients who were healthy enough to tolerate surgery with minimal distant metastatic disease. Unfortunately, NCDB does not capture this information which makes this aspect difficult to evaluate. Additionally, there may also be statistical limitations given the small sample in the metastasectomy group. Thus, the role of metastasectomy still remains unclear and hardly generalizable. The decision on whether a patient with stage IV CC receives extensive surgery should be based on the clinical characteristics of the individual and a multidisciplinary approach (13).
Noticeable findings were found with regards of multiple demographic characteristics. Patient with higher level of education who resided in a metropolitan area had better survival outcomes in the overall cohort, while higher income, being covered by a private insurance and receiving care in an academic center were associated with lesser risk of death in both overall and surgical cohorts. This highlights the potential presence of healthcare disparities for patients being treated for stage IV CC and finds confirm in the current literature (24,25). Patients with those characteristics may in fact have an easier access to healthcare, specialized surgeons and/or more technically advanced centers (26). The healthcare disparities phenomenon has been extensively studied in recent years for multiple settings and conditions (27-29). However, it still represents a major concern as certain patients may receive suboptimal treatments, develop more complications, and be at risk of worse survival outcomes.
Limitations of this study include its retrospective design, with the biases therein. A main limitation is the lack of granularity, with special regards to the number of metastases and the type of performed procedures. The NCDB does not record information about the number of metastases and their location within the same organ, as well as it does not include what type of surgical procedure was used for the metastasis resection. This represents barrier for classifying patients affected by either resectable or unresectable disease, and therefore for their selection. Ultimately, some risk factors were not included in the multivariate analyses because they were not statistically significant in the univariate model. Nonetheless, to our knowledge this study included the analysis of surgical outcomes on the largest cohort of patients diagnosed with stage IV CC.
In conclusion, the results of this study support PTR in select patients diagnosed with metastatic CC, as it provides a remarkable improvement to survival rate. The role of metastasectomy remains however controversial and no difference in survival outcomes exists between patients who received it and who did not. Ultimately, we report the presence of potential healthcare disparities as patients with higher income, education and better insurance status are more likely to survive. More research and especially prospective studies are needed to better understand these critical aspects, in order to provide systematized strategies for stage IV CC patients undergoing surgery.
Acknowledgments
We acknowledge and thank the American College of Surgeons Committee on Cancer for providing access to the Participant User File from the National Cancer Data Base.
Funding: This work was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) grant P30CA016056.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. As this study utilized a nationwide, de-identified database, it was deemed exempt from the Institutional Review Board.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149:659-69. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med 2002;137:129-31. [Crossref] [PubMed]
- Robinson JR, Newcomb PA, Hardikar S, et al. Stage IV colorectal cancer primary site and patterns of distant metastasis. Cancer Epidemiol 2017;48:92-5. [Crossref] [PubMed]
- Ba-Sang DZ, Long ZW, Teng H, et al. A network meta-analysis on the efficacy of sixteen targeted drugs in combination with chemotherapy for treatment of advanced/metastatic colorectal cancer. Oncotarget 2016;7:84468-79. [Crossref] [PubMed]
- Botrel TEA, Clark LGO, Paladini L, et al. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer 2016;16:677. [Crossref] [PubMed]
- Li Z, Huang Y, Zhao R, et al. Chemotherapy plus Panitumumab Versus Chemotherapy plus Bevacizumab in Metastatic Colorectal Cancer: A Meta-analysis. Sci Rep 2018;8:510. [Crossref] [PubMed]
- Paolo M. Adjuvant chemotherapy for high-risk stage II and stage III colon cancer: timing of initiation and optimal duration. J buon 2018;23:568-73. [PubMed]
- Hu CY, Bailey CE, You YN, et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg 2015;150:245-51. [Crossref] [PubMed]
- Shapiro M, Rashid NU, Whang EE, et al. Trends and predictors of resection of the primary tumor for patients with stage IV colorectal cancer. J Surg Oncol 2015;111:911-6. [Crossref] [PubMed]
- Chua TC, Morris DL. Therapeutic potential of surgery for metastatic colorectal cancer. Scand J Gastroenterol 2012;47:258-68. [Crossref] [PubMed]
- Logan JK, Huber KE, Dipetrillo TA, et al. Patterns of care of radiation therapy in patients with stage IV rectal cancer: a Surveillance, Epidemiology, and End Results analysis of patients from 2004 to 2009. Cancer 2014;120:731-7. [Crossref] [PubMed]
- Vogel JD, Eskicioglu C, Weiser MR, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Colon Cancer. Dis Colon Rectum 2017;60:999-1017. [Crossref] [PubMed]
- de Mestier L, Neuzillet C, Pozet A, et al. Is primary tumor resection associated with a longer survival in colon cancer and unresectable synchronous metastases? A 4-year multicentre experience. Eur J Surg Oncol 2014;40:685-91. [Crossref] [PubMed]
- Massarweh NN, Li LT, Sansgiry S, et al. Primary Tumor Resection and Multimodality Treatment for Patients with Metastatic Colon Cancer. Ann Surg Oncol 2016;23:1815-23. [Crossref] [PubMed]
- Karoui M, Roudot-Thoraval F, Mesli F, et al. Primary colectomy in patients with stage IV colon cancer and unresectable distant metastases improves overall survival: results of a multicentric study. Dis Colon Rectum 2011;54:930-8. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Hanna MH, Hwang G, et al. Outcomes of colon resection in patients with metastatic colon cancer. Am J Surg 2016;212:264-71. [Crossref] [PubMed]
- Alawadi Z, Phatak UR, Hu CY, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer 2017;123:1124-33. [Crossref] [PubMed]
- Kemeny NE, Chou JF, Boucher TM, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol 2016;113:477-84. [Crossref] [PubMed]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [Crossref] [PubMed]
- Salah S, Ardissone F, Gonzalez M, et al. Pulmonary metastasectomy in colorectal cancer patients with previously resected liver metastasis: pooled analysis. Ann Surg Oncol 2015;22:1844-50. [Crossref] [PubMed]
- Shah SA, Haddad R, Al-Sukhni W, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg 2006;202:468-75. [Crossref] [PubMed]
- Hishida T, Tsuboi M, Okumura T, et al. Does Repeated Lung Resection Provide Long-Term Survival for Recurrent Pulmonary Metastases of Colorectal Cancer? Results of a Retrospective Japanese Multicenter Study. Ann Thorac Surg 2017;103:399-405. [Crossref] [PubMed]
- Neuwirth MG, Epstein AJ, Karakousis GC, et al. Disparities in resection of hepatic metastases in colon cancer. J Gastrointest Oncol 2018;9:126-34. [Crossref] [PubMed]
- Ratnapradipa KL, Lian M, Jeffe DB, et al. Patient, Hospital, and Geographic Disparities in Laparoscopic Surgery Use Among Surveillance, Epidemiology, and End Results-Medicare Patients With Colon Cancer. Dis Colon Rectum 2017;60:905-13. [Crossref] [PubMed]
- Pulte D, Jansen L, Brenner H. Disparities in Colon Cancer Survival by Insurance Type: A Population-Based Analysis. Dis Colon Rectum 2018;61:538-46. [Crossref] [PubMed]
- Gabriel E, Narayanan S, Attwood K, et al. Disparities in major surgery for esophagogastric cancer among hospitals by case volume. J Gastrointest Oncol 2018;9:503-16. [Crossref] [PubMed]
- Osagiede O, Spaulding AC, Cochuyt JJ, et al. Disparities in minimally invasive surgery for colorectal cancer in Florida. Am J Surg 2019;218:293-301. [Crossref] [PubMed]
- Rodriguez-Vila O, Nuti SV, Krumholz HM. Healthcare Disparities Affecting Americans in the US Territories: A Century-Old Dilemma. Am J Med 2017;130:e39-42. [Crossref] [PubMed]