
Dose-modified gemcitabine plus nab-paclitaxel front-line in advanced pancreatic ductal adenocarcinoma with baseline hyperbilirubinemia
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for 80–90% of pancreatic cancers (1). Maintaining a dismal prognosis, PDAC represents the third most common cause of cancer-related deaths common amongst both men and women in the United States with an overall 5-year survival rate of ~5–8% (1-3). The 5-year overall survival (OS) rate declines further to <3% in the advanced setting (4). By 2030, pancreatic cancer is projected to be the second most fatal malignancy (5).
In 1997, gemcitabine (GEM) monotherapy was established as the standard front-line therapy with modest survival outcomes for metastatic patients (6). Various GEM-based chemotherapy combinations were evaluated following monotherapy approval; however, these studies failed to define a clinically utilized standard combination. Therefore, in 2011, Conroy et al. provided a pivotal study altering metastatic PDAC treatment (7). They reported on a phase II-III multicenter, randomized controlled trial comparing a fluoropyrimidine combination regimen of 5-fluorouracil + leucovorin + oxaliplatin + irinotecan (FOLFIRINOX) to GEM alone. Median OS and progression-free survival (PFS) were improved in the FOLFIRINOX arm with a median OS of 11.1 months and PFS of 6.4 months reported in the FOLFIRINOX arm compared to median OS of 6.8 months and PFS of 3.3 months in the GEM arm (P<0.001). Shortly after these improved outcomes with FOLFIRINOX over GEM, Von Hoff et al. (MPACT trial) reported the results of a multicenter phase III randomized trial comparing GEM + nab-paclitaxel (NabP) to GEM alone in metastatic PDAC (8). The combination regimen was dosed giving NabP at 125 mg/m2 intravenous (IV) followed by GEM 1,000 mg/m2 IV over 30 minutes both given days 1, 8, and 15 every 4 weeks. Similar to FOLFIRINOX, the combination regimen improved outcomes compared to GEM alone. Median OS was 8.5 months in the combination arm compared to 6.7 months in the GEM monotherapy arm (P<0.001). Median PFS showed an almost 2 months improvement with median PFS at 5.5 months in the combination arm compared to 3.7 months in the monotherapy arm (P<0.001). In the combination group, 41% of patients required a reduction in the NabP dose, and 47% required a GEM dose reduction. Neutropenia and leukopenia were the most frequently noted grade ≥3 adverse events in the combination arm.
Both combination regimens (FOLFIRINOX and NabP + GEM) are incorporated currently in front-line treatment of locally advanced/metastatic PDAC (1,2). Von Hoff et al.’s pivotal MPACT trial excluded patients with an elevated baseline total bilirubin (8). Further, NabP prescribing information lacks a starting dose recommendation for PDAC patients with hepatic impairment [AST <10× upper limit of normal (ULN) and total bilirubin >1.5× ULN] stating “not recommended” in these situations (9). The purpose of this retrospective review was to review the safety and efficacy of GEM-NabP in patients with baseline hyperbilirubinemia.
Methods
We performed a single institution, retrospective chart review of patients with clinically borderline resectable, locally advanced, or metastatic PDAC who received GEM-NabP front-line. Adult patients who initiated this regimen during July 01, 2013–July 01, 2017 were reviewed for baseline hyperbilirubinemia (total bilirubin >2 mg/dL) at start of therapy. Patients were included if they were seen at our center along with radiographic evaluation follow-up. OS was our primary objective. Secondary objectives were time on treatment (TOT) and response defined as disease control (any response + stable disease) vs. progression on first radiographic evaluation. Further outcomes were dosing practices, delays, any grade 3/4 hematologic toxicities, admissions, adverse effects, and reasons for stopping treatment.
Data collection included patient demographics and tumor characteristics. These factors were age, gender, clinical disease stage (borderline resectable, locally advanced, or metastatic), and location of primary tumor (head; tail; body). Baseline total bilirubin value, stent placement (yes; no), start date of GEM-NabP treatment along with doses, chemotherapy schedule used, cycle number when doses were escalated, and number of cycles received were reported. Additionally, date of progression, date of death, or last follow-up were collected. Number of treatment delays, reason for delay, hospital admissions related to toxicity, need for growth factor support, and adverse effects (per grade) along with reasons for stopping GEM-NabP (radiographic progression, clinical progression, performance status decline, and/or toxicity) were collected.
Ethics
Our study was approved by the institutional review board and a waiver of consent was granted due to patients lost to follow-up, no longer at the institution, or expired. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work area appropriately investigated and resolved.
Statistical analysis
Statistical analysis was performed using descriptive statistics. Continuous variables were described using median and range while categorical data was summarized using frequencies and percentages. OS, the primary objective, was calculated as start of GEM-NabP to death or last follow-up date. TOT was defined as treatment start date to treatment discontinuation for progressive disease, performance status decline, toxicity, patient preference, transition to local therapy for locally advanced patients, or death/last follow-up. Disease control was defined as any response + stable disease at first radiographic scan. Common Terminology Criteria for Adverse Effects (CTCAE) version 4 was utilized to determine adverse effect grade retrospectively when applicable.
Results
Twelve total patients were included in the analysis with a median age of 71 years old (range, 56–81 years old) with a 50:50 split on gender. Overall demographics are reported in Table 1. Fifty-eight point three percent were metastatic. Most patients’ (75%) primary pancreatic tumor was located in the head of the pancreas. Median baseline bilirubin was 2.4 mg/dL. The median number of cycles given was eight (range, 1–27 cycles). Each cycle was given every 2 weeks or on a 3 week on 1 week off schedule. Most (75%) received this in a biweekly fashion. Median doses reported were NabP 100 mg/m2 IV (range, 65–125 mg/m2) plus GEM 600 mg/m2 (range, 500–600 mg/m2) given at a fixed dose rate (10 mg/m2/min). Of note, standard practice at our institution is to administer gemcitabine in a fixed-dose rate with doses ranging from 600 to 750 mg/m2. The hyperbilirubinemia case in most cases (n=11) was biliary obstruction. These cases received biliary stent placement to relieve obstruction. One patient had extensive liver metastases unable to have a biliary stent placed to relieve obstruction. Forty-one point seven percent of patients had doses escalated as bilirubin elevations subsided. The median cycle number for dose escalation to full NabP dose (125 mg/m2) was cycle two (range cycle number 2–8). Neither of the borderline resectable patients were able to proceed with surgery. All three locally advanced patients received capecitabine plus radiation therapy after stabilization of disease on GEM + NabP.
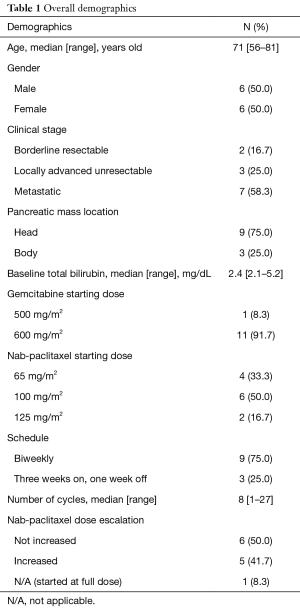
Full table
Outcomes are listed in Table 2. Median OS, TOT, and disease control were 13.9, 5.2 months, and 58%, respectively. One patient had yet to progress at time of data collection. Most patients stopped therapy due to radiographic or clinical progression. The three locally advanced patients stopped GEM + NabP to proceed with consolidative chemoradiation. One patient stopped therapy due to a decline in performance status and toxicity. Focusing on only those with metastatic disease (n=7), clinical outcomes were less favorable (median OS =6.9 months; median TOT =2.1 months, 28% disease control rate on first scan).
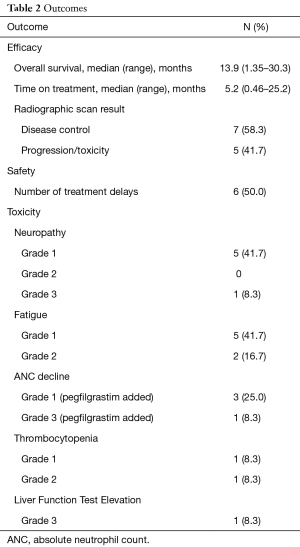
Full table
Fifty percent of all patients required a dose delay; however only 41.7% required a dose delay attributed to chemotherapy adverse effects. Two patients were admitted due to infection unrelated to chemotherapy toxicity and were not neutropenic at the time. All but one patient had a biliary stent placed to relieve dilation/obstruction. The one patient without a stent placed stopped treatment due to grade 3 liver function elevation. Reasons for dose delays were grade 2 fatigue (2 patients), grade 3 neutropenia (1 patient), grade 1 thrombocytopenia (1 patient), grade 2 thrombocytopenia (1 patient), and reports of fever and chills (1 patient). Toxicities are listed in Table 2. The median absolute neutrophil nadir and platelet nadir were 2.17 k/uL (range, 0.71–4.76 k/uL) and 138 k/uL (range, 54–242 k/uL). Patients with delays due to thrombocytopenia had platelet count recovery in time for the planned next treatment and the patient delayed due to grade 3 neutropenia recovered counts after 7 days. Secondary growth factor prophylaxis was given to this patient for future cycles. Most frequent adverse effects were grade 1 fatigue and grade 1 neuropathy.
Discussion
Advanced PDAC patients with baseline hyperbilirubinemia were excluded in the pivotal PDAC phase III trials that have established current practice (7,8). FOLFIRINOX represents a challenge for these patients, as prescribing information for irinotecan does not recommend its use in patients with a total bilirubin >2 mg/dL (10). Performance status in this population may additionally represent a barrier to FOLFIRINOX administration. Gemcitabine prescribing information lacks guidance with dosing in this setting; however, literature has provided recommended adjustments (11-13). Taxanes are primarily hepatically metabolized which raises safety concerns when prescribing these agents in the presence of hepatic dysfunction due to potential prolonged clearance and increased systemic exposure (12-15). Dose adjustments or taxane avoidance to avoid myelosuppression and treatment-related death are suggested based on limited data.
Existing safety and efficacy data for PDAC patients with hyperbilirubinemia utilizing GEM + NabP is limited. Our study showed a 41.7% dose delay due to chemotherapy. Reasons for dose delays (i.e., neutropenia, thrombocytopenia, and fatigue) in our small subset of patients were similar to those in the MPACT study in which dose delays were frequent (71% ≥1 NabP dose delay) (16). Phase I pharmacokinetic safety study evaluating GEM+ NabP in PDAC patients with cholestatic hyperbilirubinemia unfortunately was terminated early due to infrequent enrollment (17). A recent retrospective analysis by Pelzer et al., reported 29 patients with advanced PDAC treated with Gem + NabP with hyperbilirubinemia (total bilirubin ≥1.2 mg/dL) (18). Similar to our study, patients in this analysis were mostly metastatic and had their primary tumor in the head of the pancreas. The majority (68%) of patients received Gem + NabP in the first or second-line setting. The remainder received GEM + NabP in the third or fourth-line setting. Most patients (62%) had a total bilirubin ≥1.2–3 mg/dL. Fourteen percent had total bilirubin >3–5 mg/dL and 24% had a total bilirubin >5 mg/dL. The majority of patients received full dose of GEM (1,000 mg/m2) + NabP (125 mg/m2) 3 weeks on 1 week off. The authors did not identify any unexpected toxicities. They concluded that GEM + NabP did not cause severe early toxicity in patients with hyperbilirubinemia. Vogel et al. provided a recent review and German expert opinion regarding GEM + NabP use in PDAC patients with hyperbilirubinemia (19). The panel concluded that hyperbilirubinemia is a result of multiple types of hepatic dysfunction and the etiology should be determined prior to starting therapy. The panel suggested reduced starting doses for GEM + NabP based on the degree of hyperbilirubinemia coupled with the reason for hepatic impairment.
Conclusions
Despite our limited sample size our data adds to the available literature recognizing the safe administration of GEM + NabP in PDAC patients with hyperbilirubinemia at baseline bilirubin ranging from 2.1–5.2 mg/dL. Median doses were NabP 100 mg/m2 + GEM 600 mg/m2, administered at fixed dose rate (10 mg/m2/minute) given biweekly or in a 3 weeks on 1 week off fashion. We recommend determining the underlying etiology for hyperbilirubinemia with attempts to correct the underlying cause prior to GEM + NabP if possible, and we encourage continued reporting in this area to determine efficacy in this patient population. We recommend a cautious dose modification and close monitoring approach with these patients until more data is available.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ducreux M, Cuhna A, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol 2015;26 Suppl 5:v56-68. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma. Version 2. 2018. Accessed 9/28/18.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Pancreatic Cancer. Accessed 9/28/18.
- Chiaravalli M, Reni M, O’Reilly EM. Pancreatic ductal adenocarcinoma: state-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev 2017;60:32-43. [Crossref] [PubMed]
- Adamska A, Domenichini A, Falasca M. Pancreatic ductal adenocarcinoma: current and evolving therapies. Int J Mol Sci 2017. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Abraxane (paclitaxel protein-bound particles for injectable suspension) prescribing information. Celgene Corporation. Summit NJ. 2018 August.
- Camptosar (irinotecan) prescribing information. Pfizer. New York, NY. 2018 August.
- Gemzar (gemcitabine) prescribing information. Indianapolis, IN. Eli Lilly and Company. 2018 May.
- Floyd J, Mirza I, Sachs B, et al. Hepatotoxicity of chemotherapy. Semin Oncol 2006;33:50-67. [Crossref] [PubMed]
- Superfin D, Iannucci AA, Davies AM. Commentary: oncologic drugs in patients with organ dysfunction: a summary. Oncologist 2007;12:1070-83. [Crossref] [PubMed]
- Taxol (paclitaxel) prescribing information. Princeton, NJ. Bristol-Myers Squibb Company. 2011 April.
- Taxotere (docetaxel) prescribing information. Bridgewater, NJ. Sanofi-Aventis. 2010 May.
- Scheithauer W, Ramanathan RK, Moore M, et al. Dose modification and efficacy of nab-paclitaxel plus gemcitabine vs. gemcitabine for patients with metastatic pancreatic cancer: phase III MPACT trial. J Gastrointest Oncol 2016;7:469-78. [Crossref] [PubMed]
- Clinicaltrials.gov. Pharmacokinetic and safety study of nab®-paclitaxel (ABI-007) plus gemcitabine in subjects with advanced pancreatic cancer who have cholestatic hyperbilirubinemia. Available online: https://clinicaltrials.gov/ct2/show/NCT02267707. Accessed 9/28/18.
- Pelzer U, Wislocka L, Jühling A, et al. Safety and efficacy of Nab-paclitaxel plus gemcitabine in patients with advanced pancreatic cancer suffering from cholestatic hyperbilirubinaemia-a retrospective analysis. Eur J Cancer 2018;100:85-93. [Crossref] [PubMed]
- Vogel A, Kullmann F, Kunzmann V, et al. Patients with advanced pancreatic cancer and hyperbilirubinaemia: review and german expert opinion on treatment with nab-paclitaxel plus gemcitabine. Oncol Res Treat 2015;38:596-603. [Crossref] [PubMed]


