The role of chemotherapy and/or octreotide in patients with metastatic gastroenteropancreatic and hepatobiliary neuroendocrine carcinoma
Introduction
Neuroendocrine tumors (NETs) are composed of a heterogeneous group of malignancies derived from neuroendocrine cell compartments, with roles in both the endocrine and the nervous system. The majority of NETs are gastroentero-pancreatic (GEP) in origin, arising in the foregut, midgut, or hindgut (1). It has been known that NETs are very rare disease (2). However, recent studies on NET based on the Surveillance, Epidemiology and End Results (SEER) cancer registry and European studies demonstrated an increasing rate of NETs (2). Moreover, the Korean study showed a remarkable increase of the incidence of GEP-NET during the last decade (3). Whereas most NETs follow a relatively indolent course, a small percentage (9.1%) are aggressive high grade tumors with poor differentiation. The characteristics of tumor whether it is indolent or aggressive is determined by tumor grade or differentiation (2,4). Generally, tumor grade or differentiation has been based on World Health Organization (WHO) classification. The 2010 WHO classification divides NETs into two main subgroups, tumor (NET) and carcinoma (NEC), according to Ki-67 value. NET is defined as tumor with <20% Ki-67, and it is further sub-classified into Grade 1 (G1) (Ki-67 ≤2) and Grade 2 (G2) (Ki-67 3-20%). All NETs with Ki-67 >20% (G3) are NECs which included small cell or large cell carcinoma (5).
Surgery is the only curative modality in localized disease. In patients with inoperable advanced disease, there have been some therapeutic challenges such as octreotide for hormonal control, cytotoxic agents (doxorubicin, streptozocin, capecitabine, dacarbazine and temozolomide), and peptide receptor radionuclide therapy.
Recently, the PROMID study confirmed anti-tumor effect of octreotide in functional and nonfunctional well differentiated metastatic midgut NETs (6). Moreover, two agents inhibiting relevant molecular targets have been approved by the U.S. Food and Drug Administration (FDA) for NET with promising outcomes (7,8). However, these therapeutic challenges were evaluated for mainly advanced stage with G1 or G2 based 2010 WHO classification (6-8). As different from NET, there has been still the limited therapeutic option for GEP-NEC. As the clinical behavior of GEP-NEC is similar to that of small cell lung cancer (SCLC) known to be responsive to etoposide and cisplatin (EP) (9), EP has been the most widely used combination in extra-pulmonary NEC including GEP-NEC. Moertel et al. and Mitry et al. reported that on subgroup analysis, EP had favorable efficacy in NEC including GEP-NEC (10,11). Based on these studies, the combination EP has been considered as the reference treatment for GEP-NEC (10-12).
GEP-NECs including hepatobiliary (HB) tract are rare and a heterogenous group of malignancies. Primary HB-NEC comprises less than 1% of all carcinoid tumors (13,14). There has been no known to systemic therapy for HB-NEC. We intended to evaluate whether octreotide in GEP-NEC affects survival outcome or not. Simultaneously, we investigated the outcome of chemotherapy for GEP-NECs including HB.
Patients and methods
Patients
We analyzed patients that were diagnosed as metastatic GEP-NEC in Korea University Anam Hospital between January 2009 and June 2012. The definition of GEP-NEC in this study was NEC arising GEP, and HB systems. The confirmation for NEC was based on the 2010 WHO classification. The following clinicopathological characteristics of all 17 patients were collected: age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), primary site, site of metastasis, the number of metastatic site, debulking operation, and liver metastasis.
Chemotherapy
The decision for administering first line chemotherapy depended, in all cases, on the discussion between physician and patient. The chemotherapy regimen to be used was determined by the treating physician. Chemotherapy was repeated every 2-4 weeks according to regimen. All tumor measurements were assessed after every two or three cycles of chemotherapy, by using computed tomography (CT) scan and other tests that were used initially to stage the tumor. Responses were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0.
Octreotide
Octreotide 30 mg was administered intramuscularly every 28 days by nurses or physicians. Treatment was continued until tumor progression or patients’ demands.
Statistical analysis
The main goal of this analysis was to evaluate whether octreotide in GEP-NEC affects survival outcome or not. Additionally, outcomes for chemotherapies in GEP-NEC were examined. Treatment outcomes were estimated as response rate (RR), disease control rate (DCR), progression free survival (PFS), and overall survival (OS). PFS and OS were defined as from the first study treatment to the date of disease progression or death, respectively. Descriptive statistics were reported as proportions and medians. Kaplan-Meier estimates were used in the analysis of all time to event variables, and the 95% confidence interval (CI) for the median time to event was computed. Significant prognostic variables in univariate analysis for OS were included in multivariate analysis. Two-tailed Pearson’s χ2 test was used to compare the percentages in the different subgroups. A P value less than 0.05 was considered statistically significant.
Results
Patients’ characteristics
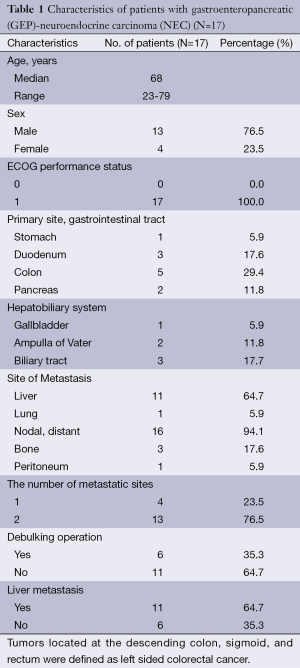
Between January 2009 and June 2012, a total of 17 patients with a diagnosis of NEC arising from the digestive system were analyzed. Baseline characteristics of the patients are listed in Table 1. The median age of patients was 68 years (range, 23-79 years) and male to female ratio was 3.25. All patients had an ECOG PS of one. The most common primary site was the colon (29.4%), followed by the duodenum and biliary tract (17.6%), pancreas and ampulla of vater (22.8) and stomach and gallbladder (5.9%). Of all patients with distant metastasis at diagnosis, lymph node was the predominant metastatic site, followed by the liver, bone and lung and peritoneum. Six of all 17 patients had debulking operation before the systemic chemotherapy.
Full table
Systemic chemotherapy
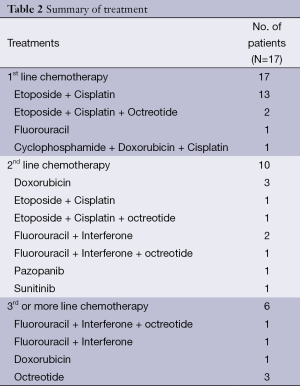
All 17 patients received systemic treatment. The most commonly used regimen as the first line therapy was EP (n=15, 88%) followed by 5-fluorouracil (n=1, 6%) and cyclophosphamide-doxorubicin-cisplatin (n=1, 6%) (Table 2). Of 15 with EP as the first line, two patients concurrently received octreotide, a somatostatin analogue. Among all 17 patients with the first line chemotherapy, one complete response and six partial responses were observed (overall RR, 41.2%). Stable disease was observed in six patients (35.3%). The DCR was 76.5% (Table 3). After the disease progression to the first line therapy, ten (58.8%) patients continued to receive the second line chemotherapy (Table 2). Doxorubicin monotherapy was used most commonly (n=3). Two patients who had not received EP as the first line were treated by EP with or without octreotide as the second line therapy.
Full table

Full table
The effect of octreotide on survival and prognosis
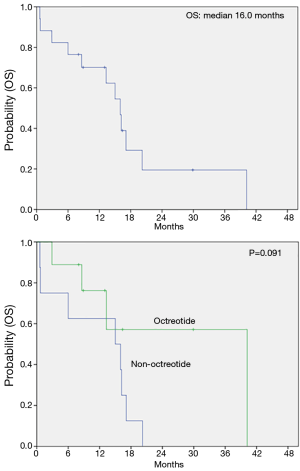
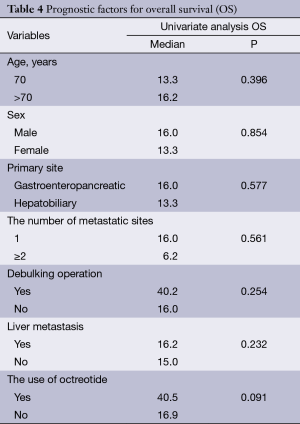
The median PFS for the first line therapy was 4.8 months. The median OS was 16 months (95% CI, 12.8-19.2) and, interestingly, the median OS in patients receiving octreotide during the treatment period was 40.2 months. We analyzed patients’ age, sex, primary site, the number of metastatic sites, debulking operation, liver metastasis and the use of octreotide to identify prognostic factors for survival. In univariate analysis, any clinico-pathologic features including sex, the location of primary tumor, the number of metastatic sites, the debulking operation and the liver metastasis did not have a major prognostic value regarding OS (Table 4). However, the use of octreotide revealed favorable trend for OS (P=0.091) (Figure 1).
Full table
Discussion
Chemotherapy is the main therapeutic option for GEP-NEC. EP combination represents the most commonly used regimen in NEC, based on similarity between NEC and SCLC (9). However, studies with this regimen are lacking in GEP-NEC unlike SCLC. Octreotide is well known to be effective in terms of syndrome control and circulating markers reduction in GEP NET (15,16). Its anti-proliferative effect is much less clear (17). Recently, a possible direct antitumor effect of octreotide on NET was proven (6). However, this effect was limited to just well differentiated NET (G1). Thus, the antitumor effect of octreotide on NEC is still unknown. Our study analyzed the effect of systemic chemotherapy in pure GEP-NEC patients. Furthermore, we evaluated whether the use of octreotide in treatment of GEP-NEC affects survival outcome or not.
This analysis showed that RR of the first line chemotherapy, usually EP was 41.2% and DCR was 76.5%. Our outcomes were consistent with several previous studies. In a French retrospective analysis of advanced NET with EP reported in 1999, RR was 42% in 41 GEP and non-GEP NEC patients (11). Fjällskog reported that the RR was 40% in poorly differentiated NET treated with EP (12). On the other hand, others were inconsistent with our outcomes. In a Japanese study on HB-pancreatic NEC treated with EP, RR was 14% (18). Moertel, et al. showed that RR was 67% in poorly differentiated NET treated with EP (10). These diverse outcomes may be caused by heterogeneous study population. Some studies included relatively many non-GEP NEC patients (10,11) and others were conducted for mainly HB-NEC patients, known as very poor prognosis (18). The population in our study was relative homogeneous. All patients with NEC arising from gastrointestinal tract (11 GEP- and 6 HB- NEC) were analyzed for this study.
Somatostatin is a peptide, the structure of which includes 14 amino acids; it has high affinity for all five types of somatostatin receptors (SSTR). Its commercially available analog, octreotide, consists of eight amino acids and binds with high affinity to SSTR. Although the role of octreotide has been unclear in NEC, octreotide monotherapy and combination of octreotide and chemotherapy have demonstrated a good efficacy in NET (6,19,20). Recently, a phase II trial for EP plus lanreotide was conducted, includeing 27 patients with non-well differentiated endocrine tumors. Ten of these patients had a GEP-NEC (21). Thirty seven percent RR and 81.5% DCR were observed. The OS was 24 months. This phase II study was the first to report the effect of chemotherapy plus somatostatin in non-well differentiated endocrine tumors. However, that included only a small number of patients with NEC, especially GEP-NEC. In our study, 9 of 17 patients with GEP- or HB- NEC received octreotide as single (n=3) or combination with chemotherapy (n=6) throughout the course of disease. The decision for administering octreotide depended, in all cases, on the discussion between physician and patients. Recently, physicians in our institute revealed the trend for concurrently using octreotide with chemotherapy. We used octreotide for the purpose of antitumor effect. The chemotherapy regimen to be used was determined by the treating physician. The median OS was 16 months (95% CI, 12.8-19.2) and the median OS in patients receiving octreotide during treatment period was 40.2 months. In addition, univariate analysis showed that the use of octreotide during the course of disease offered favorable trend for OS.
Our study was retrospective analysis with small sample size. Also, octreotide was used unsystematically and we did not evaluate the various biologic characteristics in all patients, for example, the level of vascular endothelial growth factor (VEGF) and the degree of expression for SSTR subtype (22-25). The difference of these biologic characteristics may influence on treatment outcome for octreotide or cytotoxic chemotherapy. Nevertheless, this analysis identified the effect of systemic chemotherapy in pure GEP- or HB- NEC patients and evaluated whether octreotide in GEP-NEC affects survival outcome or not. These data may provide useful information and background for future research on GEP-NEC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Williams ED, Sandler M. The classification of carcinoid tum ours. Lancet 1963;1:238-9. [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [PubMed]
- Gastrointestinal Pathology Study Group of Korean Society of Pathologists, Cho MY, Kim JM, et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat 2012;44:157-65. [PubMed]
- Pape UF, Berndt U, Müller-Nordhorn J, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2008;15:1083-97. [PubMed]
- Bernick PE, Klimstra DS, Shia J, et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum 2004;47:163-9. [PubMed]
- Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [PubMed]
- Moertel CG, Kvols LK, O’Connell MJ, et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227-32. [PubMed]
- Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer 1999;81:1351-5. [PubMed]
- Fjällskog ML, Granberg DP, Welin SL, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer 2001;92:1101-7. [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [PubMed]
- Chamberlain RS, Blumgart LH. Carcinoid tumors of the extrahepatic bile duct. A rare cause of malignant biliary obstruction. Cancer 1999;86:1959-65. [PubMed]
- Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev 1991;12:450-82. [PubMed]
- Arnold R, Simon B, Wied M. Treatment of neuroendocrine GEP tumours with somatostatin analogues: a review. Digestion 2000;62:84-91. [PubMed]
- Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer 1993;72:244-8. [PubMed]
- Iwasa S, Morizane C, Okusaka T, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol 2010;40:313-8. [PubMed]
- di Bartolomeo M, Bajetta E, Buzzoni R, et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer 1996;77:402-8. [PubMed]
- Brizzi MP, Berruti A, Ferrero A, et al. Continuous 5-fluorouracil infusion plus long acting octreotide in advanced well-differentiated neuroendocrine carcinomas. A phase II trial of the Piemonte oncology network. BMC Cancer 2009;9:388. [PubMed]
- Correale P, Sciandivasci A, Intrivici C, et al. Chemo-hormone therapy of non-well-differentiated endocrine tumours from different anatomic sites with cisplatinum, etoposide and slow release lanreotide formulation. Br J Cancer 2007;96:1343-7. [PubMed]
- La Rosa S, Uccella S, Finzi G, et al. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: correlation with microvessel density and clinicopathologic features. Hum Pathol 2003;34:18-27. [PubMed]
- Kumar M, Liu ZR, Thapa L, et al. Anti-angiogenic effects of somatostatin receptor subtype 2 on human pancreatic cancer xenografts. Carcinogenesis 2004;25:2075-81. [PubMed]
- Li M, Fisher WE, Kim HJ, et al. Somatostatin, somatostatin receptors, and pancreatic cancer. World J Surg 2005;29:293-6. [PubMed]
- Ruan W, Fahlbusch F, Clemmons DR, et al. SOM230 inhibits insulin-like growth factor-I action in mammary gland development by pituitary independent mechanism: mediated through somatostatin subtype receptor 3? Mol Endocrinol 2006;20:426-36. [PubMed]