
Patterns of care and outcomes for adjuvant treatment of pT3N0 rectal cancer using the National Cancer Database
Introduction
Colorectal cancer (CRC) is the 3rd most common cause of cancer in the United States (US), with incidence of 135,430 cases in 2017, of which 39,910 are rectal cancers (1). Furthermore, CRC is the 2nd and 3rd leading cause of death in males and females, respectively. While the overall incidence in CRC in the US is declining, there has been a notable increase in incidence among young adults since the 1980s (2). Currently, the standard of care treatment for locally advanced rectal cancer is neoadjuvant chemoradiation therapy followed by surgery. However, in the era of improved preoperative imaging and total mesorectal excision (TME), the management of clinical and pathological concordant T3N0 remains controversial.
In the US, patients presenting with clinical T3N0 rectal cancer are often treated with neoadjuvant chemoradiation therapy followed by surgery. Indeed, neoadjuvant chemoradiation therapy was shown to be better tolerated, increase local control, allow for tumor downstaging and improve likelihood of sphincter-preserving therapy in the seminal German Rectal Study comparing neoadjuvant to adjuvant chemoradiation therapy in locally advanced rectal cancer (3,4). Furthermore, the Dutch Colorectal Cancer Group (Dutch CKVO 9504) conducted a trial in which short course neoadjuvant radiation therapy was compared with TME alone in patients with resectable rectal cancer, and similarly, an improvement in local control was found without any difference in overall survival (OS) (5-7). However, there is a concern that neoadjuvant therapy may overtreat patients at lower risk of recurrence with unnecessary late toxicity.
Patients with T3N0 rectal cancer have an excellent prognosis with 5-year OS 74–84%, disease free survival (DFS) 63–75%, local recurrence (LR) 5–11%, and distant relapse 13–20% (8). Therefore, there might be a subgroup of patients with T3N0 disease who might not benefit from radiation therapy, especially in the TME era. There have been a number of retrospective series that report rates of LR of 2.7–9% among T3N0 patients undergoing TME (9-12).
However, identifying patients with true T3N0 presents a unique challenge as there are high rates of discordance between clinical and pathologic staging. Indeed, Guillem et al. published a retrospective review of 188 endorectal ultrasound/MRI staged T3N0 patients treated with neoadjuvant chemoradiation therapy followed by TME and found, despite combined modality therapy, 22% of patients had nodal positive disease (13). In another study by Lombardi et al. the rate of nodal positivity after neoadjuvant chemoradiation therapy in patients with clinical T3N0 disease was 28% (14). Similarly, in the German Rectal Study, only 25% of pre-operative group had positive nodal disease in comparison to 40% in the post-operative group. However, in the German Rectal Study, 18% of postoperative patients were over-staged and were considered not eligible for any adjuvant therapy following surgical resection.
Nevertheless, for those patients who receive upfront surgery with TME and are found to have pT3N0 disease, the optimal adjuvant treatment regimen remains to be described. There are no prospective randomized controlled studies that address this cohort of patients. Therefore, given the paucity of data available, we conducted this retrospective observational study utilizing the National Cancer Database (NCDB) for rectal cancer treated between 2004 and 2014 to determine patterns of care and outcomes for pT3N0 patients.
Methods
Data source
The NCDB is an oncological database that captures incident cancer data from >1,500 Commission on Cancer (COC)-accredited facilities nationwide. The database includes >70% of newly diagnosed cancer cases and captures a number of variables, including: demographics, tumor staging, course of treatment. Moreover, all patient information in the NCDB is de-identified. Therefore, this study was exempt from institutional review board evaluation.
Statistics
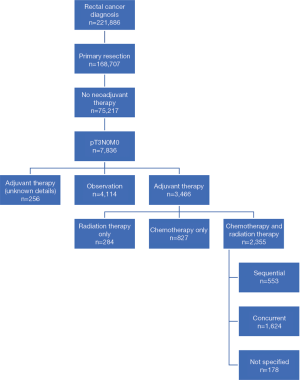
A total of 221,886 patients were identified from the NCDB with an International Classification of Disease for Oncology, 3rd Edition (ICD-O-3) site code of C209, corresponding to rectal cancer (Figure 1). We first identified the cohort who underwent surgery for their rectal cancer (N=168,707). Next, we included cases in which no neoadjuvant therapy was administered (N=75,217). Finally, we selected patients with non-metastatic and pT3N0 disease, which comprised the final target population of interest (N=7,836). Using this cohort of patients, we then determined the proportion of patients who received no additional treatment (N=4,114) and any adjuvant therapy (N=3,466), with the remaining with unreported details excluded from further analysis (N=256). Among the adjuvant therapy subgroup, we further stratified based on chemotherapy alone (N=827), radiation alone (N=284) and chemotherapy and RT (N=2,355) (Figure 2A). In order to better describe the adjuvant chemotherapy and RT subgroup, we defined concurrent chemoRT (N=1,624) as chemotherapy starting within the median RT treatment time (40 days), with sequential chemotherapy and RT (N=553) or unknown (N=178) for treatment not meeting this definition (Figure 2B).
The NCDB contains information on OS only. Therefore, all end points in this study relate to impact on OS. Univariate analysis of patient characteristics and OS was performed using the Kaplan-Meier method, with the log rank method (Mantel-Cox) to assess for significance. Factors included in the analysis are listed in Table 1.
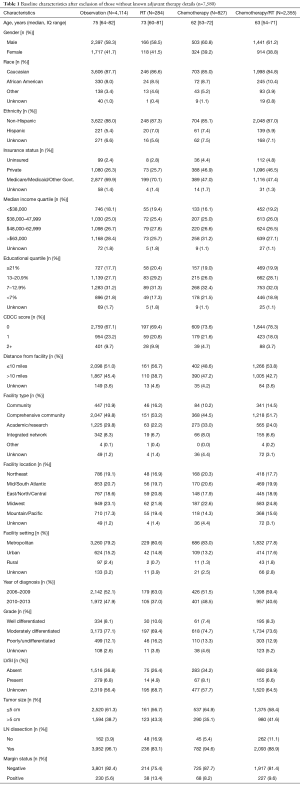
Full table
Characteristics associated with OS were evaluated in univariate fashion using log-rank testing. Based upon the sample size as a means to reduce under-capturing influential factors, statistically significant or near-significant (P<0.10) were incorporated in Multivariable Cox proportional hazards regression modeling using backward stepwise methodology. Cases with missing data were excluded from regression analyses. The receipt of adjuvant therapy, compared to observation, introduces an inherent bias by virtue of the fact that patients must live a certain period of time following primary therapy in order to receive adjuvant therapy. Therefore, to mitigate the risk of immortal time bias, a conditional landmark analysis was thereafter conducted using a cutoff of 3 months or more of follow-up.
To better account for selection bias, propensity scores were calculated for various treatment arm utilization (15,16). Scores were validated through measurement of absolute standardized differences between arms (<0.10) within designated equally distributed quintiles (17-19). Inverse probability of treatment weighting (IPTW) was then used to better estimate the effect of radiation therapy utilization, entering this as a covariate in the final regression model (20). Sensitivity analyses were conducted on the final Cox model within each propensity score quintile, verifying the findings below (21).
Results
Patient and treatment characteristics
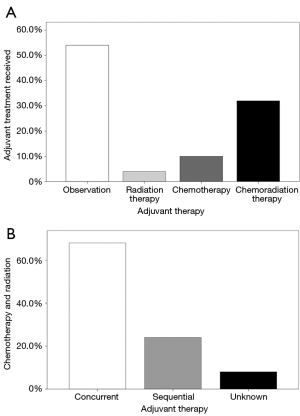
Baseline characteristics of the patients included in this study are shown in Table 1. Of the 7,580 patients with nonmetastatic, pT3N0 rectal cancer who did not receive neoadjuvant therapy and had known adjuvant therapy, there was a nearly equal proportion of patients who went on to observation (54.3%) and adjuvant therapy (45.7%). Of those patients receiving adjuvant therapy, 8.2% received RT alone, 23.9% received chemotherapy alone, and 67.9% received chemotherapy and RT (Figure 2A). Of note, chemotherapy and RT were delivered concurrently in 69.0% of patients (Figure 2B). The median time from diagnosis to initiation of treatment was 84 days (IQ 55–126 days). Among the patients receiving radiation therapy, the median dose was 50.4 Gy (IQ 45–50.4 Gy) and the median elapsed days for RT was 40 days (IQ 38–45 days).
Treatment outcomes
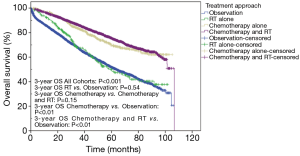
With a median follow-up of 38.6 months, the mean unadjusted OS was 61.9, 62.5, 81.0 and 83.4 months for observation, RT, chemotherapy and chemotherapy with RT. The 3-year unadjusted OS was 66.7%, 67.4%, 83.3%, and 86.0% for observation, RT alone, chemotherapy alone and chemotherapy with RT, respectively (P<0.001) (Figure 3). Moreover, there was no difference in 3-year unadjusted OS between observation and RT alone (P=0.54) or chemotherapy alone and chemotherapy with RT (P=0.15). There was a significant improvement in OS in patients that received adjuvant chemotherapy (P<0.01) or RT with chemotherapy (P<0.01) when compared to observation alone.
Univariate analysis
The complete list of factors evaluated by univariate analysis are shown in Table 2. Demographic variables that had a significant impact on OS include: age, sex, race, insurance status, median income, distance from facility, facility type, and facility location. Patient specific variables that impacted OS include: CDCC score, histologic grade, primary tumor size, lymph node dissection and margin status. Treatment variables that significantly impacted OS include: treatment approach, receipt of RT, receipt of chemotherapy, and timing of chemotherapy in relation to RT. Ethnicity, education level, metropolitan or urban setting, year of diagnosis, and the presence of LVSI were not statistically significant.
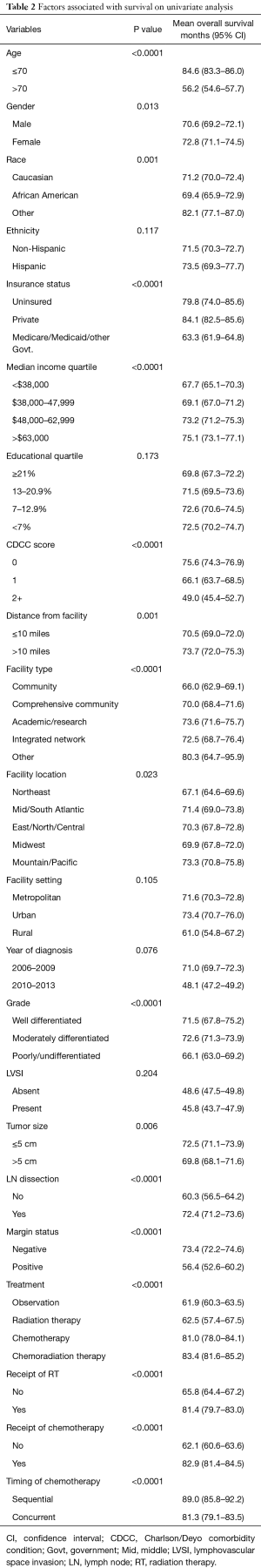
Full table
Multivariable analysis
The results of multivariable cox regression analysis, with a 3-month conditional landmark, are shown in Table 3. Demographic variables that impacted OS were: age >70 [HR 2.05 (1.82–2.31), P<0.0001], female sex [HR 0.87 (0.79–0.96), being uninsured (reference, P<0.0001)], higher median income: 3rd quartile [HR 0.78 (0.68–0.90), P=0.001] or 4th quartile [HR 0.76 (0.66–0.88) P<0.0001], and facility location: NE (reference, P=0.02). Mid/South Atlantic [HR 0.84 (0.72–0.98), P=0.027) or Mountain/Pacific [HR 0.81 (0.69–0.96) P=0.013]. Interestingly, race, distance from facility, facility type and year of diagnosis, did not impact OS in the final mode l.
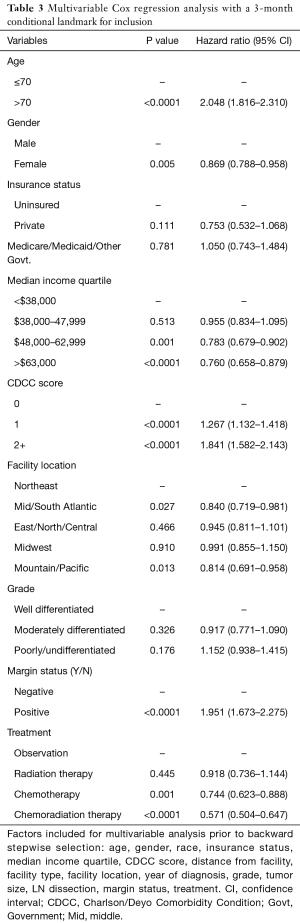
Full table
Patient specific variables with significant impact on OS include: CDCC score: 1 [HR 1.27 (1.13–1.42) P<0.0001] or 2+ [HR 1.84 (1.58–2.14) P<0.0001], well-differentiated tumors (reference, P=0.004), and positive surgical margins [HR 1.95 (1.67–2.28) P<0.0001]. Finally, type of therapy received also impacted OS: chemotherapy alone [HR 0.74 (0.62–0.89) P=0.001] or chemotherapy and RT [HR 0.57 (0.50–0.65) P<0.0001]. Moreover, tumor size and receipt of lymph node dissection did not impact OS.
In addition, separate multivariable cox regression analyses were performed on the subset of patients that had negative surgical margins (N=5,069) and positive surgical margins (N=415). In the margin negative subset, RT alone did not impact OS [HR 0.89 (0.70–1.14) P=0.361]; however, chemotherapy alone [HR 0.74 (0.61–0.89) P=0.002] and chemotherapy with RT [HR 0.59 (0.52–0.68) P<0.0001] still had improved OS. Moreover, in the margin positive subset, only chemotherapy with RT [HR 0.44 (0.31–0.63) P<0.0001] impacted OS.
Propensity-score adjusted analysis
Propensity scores with IPTW for the receipt of radiation alone or radiation with chemotherapy were generated. A doubly robust cox regression model, adjusted for IPTW, demonstrated that the cohort of patients who received RT (either alone or with chemotherapy) was associated with reduced risk of overall mortality when compared with no RT [HR 0.48 (0.43–0.54) P<0.0001]. This benefit was maintained in the margin negative cohort [HR 0.52 (0.46–0.59) P<0.0001] and margin positive cohort [HR 0.49 (0.36–0.67) P<0.0001]. However, subset analysis revealed no difference in OS when comparing adjuvant chemoradiation therapy with chemotherapy alone [HR 0.91 (0.61–1.4) P=0.66]. Similarly, no significant difference in OS was seen among the margin negative cohort [HR 0.86 (0.55–1.3) P=0.51] or the margin positive cohort [HR 0.53 (0.16–1.8) P=0.3].
Discussion
The management of rectal cancer has evolved significantly over the last 30 years. Currently, the standard of care treatment for patients with clinical Stage II rectal cancer and above, in the US, is neoadjuvant CRT followed by TME based on the German Rectal Study (4). Moreover, the National Surgical Adjuvant Breast and Bowel Project (NSABP) R-03 trial randomized a similar population of patients to neoadjuvant CRT followed by TME or TME followed by adjuvant CRT. Although NASBP R-03 did not complete accrual, at a median follow-up of 8.4 years, there was improved DFS (64.7% vs. 53.4%) and 15% pCR rate, with no recurrence in this subgroup of patients, further supporting a neoadjuvant approach for this cohort of patients (22).
The Swedish Rectal Cancer Trial (SRCT) was a large randomized control trial that recruited patients with resectable rectal cancer and randomized them to preoperative short course RT (25 Gy in 5 fractions) followed by surgery or surgery alone (23). This is the only randomized trial in rectal cancer that showed both a statistically significant local control benefit, LRR of 9% vs. 26%, and OS benefit, 38% vs. 30%, for RT (24). The benefit of RT in terms of local control was demonstrated for Stages I-III. However, it should be noted that this study was performed in the pre-TME era, and the observed benefit of RT has been attributed to suboptimal surgery.
The concept that the rectum is encapsulated within a fibrous sheath that contains perirectal lymphatic tissue was first formally described in 19th century, and has been referred to as the “Holy Plane” of rectal surgery (25). It was not until Heald published his experience with TME for 115 patients with rectal cancer, achieving a 5-year local control of 97.4%, that the technique started to gain traction (26). Therefore, the Dutch CKVO 9504 trial included a similar cohort of patients as the SRCT and patients were randomized to preoperative short course RT (25 Gy in 5 fractions) followed by TME or TME alone. In this study, there was a local control benefit of RT with 10-year LR of 5% vs. 11%. In contrast to the SRCT, there was no difference in OS for the entire cohort. However, subgroup analysis did identify a statistically significant benefit in OS for patients with Stage III disease and negative circumferential margins (50% vs. 40%) (7). Nevertheless, this trial demonstrated that improved surgical technique with TME did not negate the observed LC benefit that was seen in the SCRT.
In the current study, patterns of care for patients with pT3N0 disease were reviewed using the NCDB database. Among the 6,767 patients identified with pT3N0 disease with no preoperative therapy given, the proportion of patients treated with adjuvant therapy was similar to those who underwent observation (47% vs. 53%, respectively). This finding is reflective of current NCCN guidelines that recommend adjuvant CRT or observation for patients with favorable pathologic findings (well-moderately differentiated, invading <2 mm mesorectum, no LVI and involving proximal rectum) (27). Interestingly, while the vast majority of patients that received adjuvant therapy were treated with adjuvant CRT (68.6%), nearly a third were treated with either RT (8.3%) or chemotherapy (23.1%) alone in the adjuvant setting. Therefore, we set out to investigate which adjuvant strategy was associated with the greatest clinical outcome using a large oncological national database.
Patients with pT3N0 rectal cancer had improved OS if they received adjuvant chemotherapy (P<0.01) or adjuvant CRT (P<0.01) when compared to patients that underwent observation. Moreover, there was no difference in OS for patients treated with adjuvant RT or observation (P=0.54) or between patients treated with adjuvant chemotherapy or adjuvant CRT (P=0.15). The 3-year unadjusted OS rates were 66.7%, 67.4%, 83.3%, and 86% for patients undergoing observation, adjuvant RT, adjuvant chemotherapy or adjuvant CRT, respectively (P<0.001). The benefit of adjuvant chemotherapy (HR 0.74, P=0.001) or adjuvant CRT (HR 0.57, P<0.001) on OS persisted on multivariable cox regression. Moreover, among patients with negative margins, RT alone offered no benefit over observation (HR 0.89, P=0.361); however, chemotherapy (HR 0.74, P=0.002) and CRT (HR 0.59, P<0.0001) were associated with improved OS. However, for patients with positive margins, only chemotherapy and CRT offered a benefit over observation (HR 0.44, P<0.0001). Indication bias was accounted for by propensity scoring with IPTW-adjusted modeling, and chemotherapy, with or without radiation, remained associated with improved OS (HR 0.48, P<0.001) and this was seen in the margin negative and positive cohort as well. These results suggest that chemotherapy plays an integral role in the survival benefits seen with adjuvant therapy, likely by a reduction in the risk of distant progression. With increasing supporting data of short-course radiation therapy (25 Gy in 5 fractions) in the neoadjuvant setting, there should still remain a focus on incorporation of chemotherapy in sequential fashion, as supported by the Polish II study (28).
Despite these compelling findings, there are several limitations of this study. Firstly, the NCDB is comprised of COC-accredited facilities, and the outcomes from these institutions may not be generalizable to all hospital systems. Secondly, despite our attempt to control for confounding variables by performing a propensity score analysis, there may be additional confounders that were not identified. Moreover, tumor location within the rectum and pretherapy prognostic imaging findings are not recorded in the NCDB and cannot be accounted for. Finally, the NCDB does not report other important clinical outcomes, including: LR, regional recurrence or distant metastases. The importance of these clinical outcomes can be appreciated in the context of adjuvant therapy offered. For example, radiation therapy would be expected to impact locoregional recurrence while chemotherapy would be expected to mitigate distant metastasis, which may impact reported OS differentially.
Recently, multiple trials are evaluating the utility of preoperative MRI to identify good prognosis stage I, II and III patients that might be adequately managed with surgery alone. The MERCURY trial identified “good” prognostic features as MRI-anticipated negative CRM and MRI-predicted T2-T3b (<5 mm from muscularis propria) disease regardless of nodal stage. For “good” prognosis T3 tumors, LR was 1.7% with a 5-year OS and DFS of 68% and 81%, respectively (29). Likewise, the Optimierte Chirurgie Und MRT (OCUM) trial used MRI to identify low-risk tumors and defined them as tumors with minimum distance of >1 mm from mesorectal fascia and those tumors in upper third of rectum, regardless of nodal stage. This low-risk group was treated with TME alone and there was a 5-year LR rate of 2.7% with 5-year DFS of 76% (30). Finally, the QuickSilver trial evaluated the safety and feasibility of utilizing specific MRI criteria to identify “good prognosis” rectal tumors prior to resection, with a primary outcome of positive CRM rate. The criteria used in this study include: distance to mesorectal fascia >1 mm, definite T2, T2/early T3 or definite T3 with <5 mm extramural depth of invasion and no or equivocal extramural venous invasion. The authors observed a CRM rate of 4.9%, and concluded that MRI may be able to adequately identify patients with “good prognosis” and this subset may not need chemoRT (31). The importance of pretherapy imaging and the evolving role of MRI in risk stratification cannot be overstated. Currently, the European Society for Medical Oncology (ESMO) guidelines for rectal cancer stratify patients based on depth of invasion beyond muscularis propria as well as primary tumor location within the rectum. Using these strata, patients with primary tumors located in the middle or high rectum with up to 5 mm depth of invasion are recommended to proceed with TME alone (32).
Conclusions
Our study demonstrates the variety of adjuvant treatment strategies that are currently utilized in the US. Moreover, after adjusting for indication bias with propensity score matching, we found that receipt of adjuvant chemotherapy or CRT improved OS when compared to observation. Although both chemotherapy and CRT have similar effects on OS there was no difference in OS appreciated between adjuvant chemotherapy alone and CRT. Therefore, patients who are found to have pT3N0 disease after TME surgery may benefit from adjuvant chemotherapy alone to maximize outcomes and minimize the morbidity associated with adjuvant CRT. However, there is likely a subgroup of pT3N0 patients that benefit from additional local therapy (e.g., R1-2 resection, close CRM, high grade, LVSI, PNI, and deep perirectal fat invasion), and adjuvant CRT may be justified in this population of patients. NCDB is deficient in recording all risk factors and therefore would not be suitable to define such population. Therefore, in patients with pT3pN0, either adjuvant chemotherapy or chemoradiation is recommended while the latter should be considered in higher risk patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst 2017. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [Crossref] [PubMed]
- van Gijn W, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-82. [Crossref] [PubMed]
- Gunderson LL, Callister M, Marschke R, et al. Stratification of rectal cancer stage for selection of postoperative chemoradiotherapy: current status. GCR 2008;2:25. [PubMed]
- Enker WE, Thaler HT, Cranor ML, et al. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 1995;181:335-46. [PubMed]
- Kim JS, Kim NK, Min BS, et al. Adjuvant radiotherapy following total mesorectal excision for stage IIA rectal cancer: is it beneficial? Int J Colorectal Dis 2010;25:1103-10. [Crossref] [PubMed]
- Park IJ, Kim HC, Yu CS, et al. Effect of Adjuvant Radiotherapy on Local Recurrence in Stage II Rectal Cancer. Ann Surg Oncol 2008;15:519-25. [Crossref] [PubMed]
- Picon AI, Moore HG, Sternberg SS, et al. Prognostic significance of depth of gross or microscopic perirectal fat invasion in T3 N0 M0 rectal cancers following sharp mesorectal excision and no adjuvant therapy. Int J Colorectal Dis 2003;18:487-92. [Crossref] [PubMed]
- Guillem JG, Díaz-González JA, Minsky BD, et al. cT3N0 Rectal Cancer: Potential Overtreatment With Preoperative Chemoradiotherapy Is Warranted. J Clin Oncol 2008;26:368-73. [Crossref] [PubMed]
- Lombardi R, Cuicchi D, Pinto C, et al. Clinically-Staged T3N0 Rectal Cancer: Is Preoperative Chemoradiotherapy the Optimal Treatment? Ann Surg Oncol 2010;17:838-45. [Crossref] [PubMed]
- Rosenbaum PR. Propensity score. In: Armitage P, Colton T, editor. Encyclopedia of biostatistics. 2nd edition. Boston, MA: John Wiley & Sons, 2005:426-4272.
- D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf 2008;17:1202-17. [Crossref] [PubMed]
- Watkins S, Jonsson-Funk M, Brookhart MA, et al. An empirical comparison of tree-based methods for propensity score estimation. Health Serv Res 2013;48:1798-817. [PubMed]
- Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001;54:387-98. [Crossref] [PubMed]
- Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656-64. [Crossref] [PubMed]
- Austin PC, Mamdani MM, Stukel TA, et al. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med 2005;24:1563-78. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Cedermark B, Dahlberg M, Glimelius B, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336:980-7. [Crossref] [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: Long Lasting Benefits From Radiotherapy on Survival and Local Recurrence Rate. J Clin Oncol 2005;23:5644-50. [Crossref] [PubMed]
- Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med 1988;81:503-8. [Crossref] [PubMed]
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Rectal Cancer NCCN Guidelines Version 3. 2018. Available online: https://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf. Accessed 11/24/, 2018.
- Ciseł B, Pietrzak L, Michalski W, et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol 2019;30:1298-303. [Crossref] [PubMed]
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753-60. [Crossref] [PubMed]
- Ruppert R, Junginger T, Ptok H, et al. Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM Rectal Cancer Trial. Br J Surg 2018;105:1519-29. [Crossref] [PubMed]
- Kennedy ED, Simunovic M, Jhaveri K, et al. Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients With "Good Prognosis" Rectal Cancer Eligible for Primary Surgery: The Phase 2 Nonrandomized QuickSilver Clinical Trial. JAMA Oncol 2019;5:961-6. [Crossref] [PubMed]
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv22-40. [Crossref] [PubMed]


