A nomogram that predicts pathologic complete response to neoadjuvant chemoradiation also predicts survival outcomes after definitive chemoradiation for esophageal cancer
Introduction
Esophageal cancer is relatively rare but deadly. Despite advances in diagnosis and treatment, the overall 5-year survival rate has improved from 3% to only 15-20% since the mid-1970s (1). Neoadjuvant chemoradiation, followed by surgery if possible, is currently the standard of care for nonmetastatic esophageal cancer, as randomized trials have shown that this therapy produces a survival benefit over surgery alone (2-5). However, even though this combined therapy has led to prolonged survival outcomes, this benefit is balanced by the risk of surgical complications, which include a postoperative death rate of 4-10% (4,6), and the risk of long-term detrimental effects from postoperative pulmonary and gastrointestinal complications (7) that can lead to lifelong deterioration in terms of gastroesophageal reflux, eating restrictions, dyspnea, and fatigue (8).
About 25-30% of patients experience pathologic complete response (pCR), the absence of residual viable tumor cells in the resected specimen, after neoadjuvant chemoradiation (3,5). A pCR after trimodality therapy is known to predict lower rates of local recurrence (9,10) and better overall survival (OS) (10,11); a pCR can also indicate long-term cure in about 20% of patients with unresected disease who receive definitive chemoradiation (12). Thus the question remains: if chemoradiation eradicates the esophageal tumor, can some patients forego surgery (and be spared the perioperative and long-term morbidity of esophageal resection), and if so, how would such patients be identified?
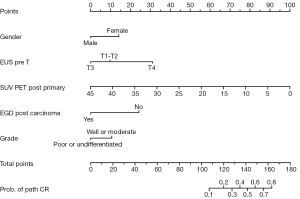
One way of addressing this question would be to develop a surrogate measure with which to identify pCR in patients who do not undergo surgery; such a surrogate could allow surgery to be reserved for use as salvage therapy if needed rather than being used in all cases. Until recently, no combinations of clinical variables, imaging findings (13-15), or biomarkers (16,17) had been identified that can accurately and reliably predict which patients will achieve a pCR. To address this need, a nomogram comprising five clinical variables was recently developed that can collectively predict pCR after trimodality therapy with ≥60% probability: (I) sex; (II) baseline T status (by endoscopic sonography); (III) tumor grade; (IV) standardized uptake values (SUVs) on positron emission tomography (PET) after chemoradiation; and (V) esophagogastroduodenoscopy (EGD)-guided biopsy findings after chemoradiation (18). For the current study, we hypothesized that this same nomogram pCR score can also predict clinical outcomes in patients treated with definitive chemoradiation alone, and our aim was to further validate this nomogram for future use in clinical decision-making.
Patients and methods
Patients
In this retrospective analysis, we identified 333 patients who received definitive chemoradiation for stage IB-IVA esophageal carcinoma at a single institution from 1998 through 2010. All patients had no evidence of distant metastases at presentation, all had received definitive concurrent chemoradiation, with or without induction chemotherapy, and all had complete information on all of the variables required for the pCR nomogram (Figure S1). The 333 patients were separated into two groups according to the median pCR nomogram score: those with score ≤125 (n=183) and those with score >125 (n=150). Disease was staged according to the 6th [2002] edition of the American Joint Committee on Cancer staging system. All analyses were approved by the appropriate institutional review board.
Chemoradiation treatment
Chemotherapy consisting of a fluoropyrimidine (IV or oral) and either a platinum compound or a taxane was given concurrently with radiation therapy to a median dose of 50.4 Gy (range, 25-66 Gy), delivered in daily 1.8-Gy fractions on Monday-Friday. Radiation was delivered by 3-dimensional conformal radiation therapy (3D-CRT), intensity-modulated radiation therapy (IMRT), or proton beam therapy (PBT) techniques. A total of 122 (36.6%) patients also received induction chemotherapy.
Nomogram score and outcome measures
As noted, the nomogram scores were based on five clinical variables: (I) sex; (II) baseline T status (by endoscopic sonography); (III) tumor grade; (IV) SUV of the primary tumor by PET after chemoradiation; and (V) EGD biopsy results after chemoradiation (18). The total number of points on the nomogram ranges from 0 to 180; in the original study, a nomogram score of >160 was found to predict pCR with ≥60% probability. However, because very few patients in our dataset had a nomogram score >160, we used the median score of the entire group of 125 as the cutoff point for our current analysis.
Dates of death were determined from the medical records and the Social Security Death Index. OS was calculated from the date of diagnosis to the date of death or last follow-up. Disease-free survival (DFS) was calculated from the date of diagnosis to the date of documented disease recurrence. Patients who had not experienced progression or recurrence or had died by the last follow-up were censored.
Statistical analysis
Data were collected retrospectively. The nomogram score was examined as a binary variable (≤125 points and >125 points) as described above. Chi-square or Fisher’s exact tests were used to compare differences between nomogram groups with respect to categorical variables. Wilcoxon rank-sum tests or Kruskal-Wallis tests were used to assess associations between nomogram group and continuous variables. Multivariable Cox regression tested the association between clinical outcomes (OS, locoregional recurrence, and distant metastasis) and patient or treatment factors and the pCR nomogram score. The individual variables for the nomogram score were not re-introduced in the multivariable analysis. Survival curves were constructed with the Kaplan-Meier method and compared between nomogram groups with the log-rank test. The clinical variables for the multivariable cox regression model were selected by backward selection with an adjusted P value ≤0.05.
Results
Patient, tumor, and treatment characteristics
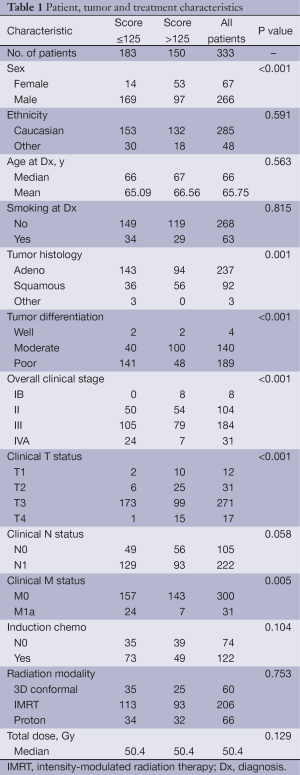
Table 1 summarizes patient, tumor, and treatment characteristics. The median age at diagnosis was 66 years; most patients were white men; most tumors were adenocarcinomas of moderate to poor differentiation; and most patients had stage II or III disease. Compared with patients with ≤125 nomogram points, patients with >125 points were more likely to be female, to have squamous cell carcinomas of well- or moderately differentiated histology, and to have lower stage disease (P for all <0.05). In terms of characteristics after chemoradiation, patients with >125 points (vs. those with ≤125) were more likely to have shown a complete response (CR) on PET/computed tomography (CT), to have had lower SUVmax values, and to have had no evidence of residual cancer cells in EGD biopsy samples obtained after chemoradiation (data not shown).
Full table
Survival outcomes
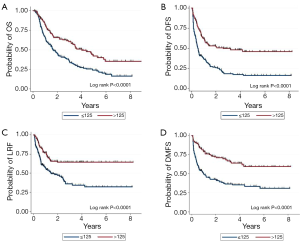
The median follow-up time for all patients was 18.2 months (30.7 months for those alive at the time of this analysis). The median OS, DFS, locoregional failure-free survival, and distant metastasis-free survival times for the entire group were 31.4, 10.7, 31.8, and 35.3 months. When patients were stratified by ≤125 vs. >125 nomogram points, the corresponding median OS times were 19.7 vs. 48.2 months, DFS times 6.1 vs. 31.1 months, locoregional failure-free survival times 17.7 months vs. not reached, and distant metastasis-free survival times 11.7 months vs. not reached (P for all <0.001) (Figure 1).
Univariate analysis
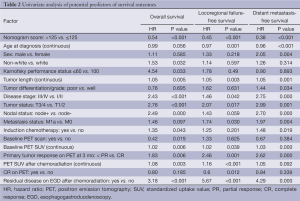
On univariate analysis, older age at diagnosis, shorter tumor length, lower overall disease stage (I/II vs. III/IV), lower baseline T status (T1/2 vs. T3/4), node-negative disease, lower baseline PET SUV, CR in the primary tumor on restaging PET/CT at 3 months after chemoradiation therapy, and absence of cancer cells on the EGD biopsy specimens obtained after chemoradiation were also associated with improved OS, locoregional failure-free survival, and distant metastasis-free survival outcomes (P<0.05). These and additional factors associated with improved locoregional-failure free survival and prolonged distant metastasis-free survival are shown in Table 2.
Full table
Multivariate analysis
Variables were selected for inclusion in multivariate analysis on the basis of their significance in the univariable analysis; other factors known to predict prognosis were included as well. As noted previously, the individual clinical variables constituting the five components of the nomogram score (sex, baseline T status, tumor grade, PET SUV and EGD biopsy results after chemoradiation) were not re-introduced in the multivariable analysis. In multivariable Cox regression analysis, the nomogram cutoff score remained an independent predictor of all survival outcomes even after adjusting for other prognostic factors (Table 3).
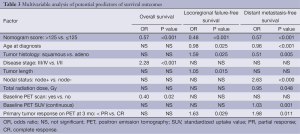
Full table
Discussion
In this study, we found that the pCR nomogram score, developed from patients treated with trimodality therapy (18), also predicted clinical outcomes in patients who did not undergo surgery. The nomogram score along with other known prognostic factors such as clinical disease stage, independently predicted OS, locoregional control, and distant metastasis-free survival. The five factors used in the pCR nomogram score seem to represent a set of clinical and tumor-specific variables that distinguish favorable from less-favorable disease, pointing to the possibility that this set of factors could help in the choice of treatment after chemoradiation.
Preoperative chemoradiation followed by surgery is currently considered the standard of care over surgery alone in the United States and elsewhere based on results of the CROSS trial (4). However, the role of surgery after chemoradiation has been controversial because of two trials that failed to show an OS benefit from the addition of surgery to chemoradiation (19,20). However, the high perioperative mortality rate in these trials (8-12%) may have obscured a survival benefit in the surgical group. Certainly esophagectomy improves local control by resecting disease that did not respond to chemoradiation; however, esophagectomy comes at the price of significant perioperative morbidity, including pulmonary, gastrointestinal, and wound-healing complications (8). It is therefore desirable to avoid surgery for patients who achieve pCR after chemoradiation, if a reliable surrogate method of identifying pCR other than surgery can be identified.
Significant effort has been expended to identify molecular biomarkers of clinical response. Molecular analyses of pretreatment biopsy specimens may help to identify tumors that will not respond well to chemoradiation; for example, two groups have shown that specimens expressing high levels of NF-kB predicted poorer pCR rates and more aggressive tumor biology (lymph node metastasis, perineural and vascular invasion) (16,21). Further, tumors that express low levels of NF-kB before therapy but higher levels after chemoradiation were also associated with poorer prognosis (22). Other investigators have found a 3-gene “signature” to predict pathologic response (17,23). However, marker studies are limited in that assessments of gene expression depend on the condition of the tissues and how they are collected and processed, and the findings thus may not be generalizable to those of other studies. Another approach using imaging-based biomarkers may circumvent potential problems with tissue collection and handling bias. For example, several groups have shown that PET SUVmax after treatment is a good predictor of pCR (13,15,24). However, a meta-analysis of several esophageal-cancer trials showed that fluorodeoxyglucose (FDG)-based PET has a predictive value of only about 70% (25). Diffusion-weighted magnetic resonance imaging (MRI), at baseline and at 2 weeks after the start of chemoradiation, has been shown to be highly accurate for predicting pathologic response (26,27). However, any biologic imaging technique will require prospective validation before it can be used to stratify patients for treatment intensification or for predicting pCR. We believe that the best predictors of response will come not from one set of marker or imaging correlates but rather from a combination of clinical and tumor response factors (as we included in the pCR nomogram), a variety of tumor-specific imaging findings, and molecular biomarkers.
The limitations of this study are the retrospective nature of the analysis and our need to restrict the analysis to patients who had information available on all five factors used to create the nomogram score. This restriction could have introduced bias in terms of excluding patients who did not have these tests because of poor condition or early death, which would have artificially improved the outcomes of the study cohort relative to all patients who received chemoradiation during the same period. Nevertheless, the nomogram score was still able to separate patients into risk groups, which underscores the robustness of this tool. Another limitation is that the factors used to build the nomogram may not be fully exportable to other centers. Some factors represent procedures that are standard at our institution, such as repeat endoscopy and biopsy after chemoradiation and repeat FDG-PET staging after treatment, but these procedures may not be considered standard practice elsewhere. Further research is needed to determine if other more generalizable factors could be used to generate a predictive nomogram.
In conclusion, the pCR nomogram score independently predicted survival outcomes after definitive chemoradiation therapy for esophageal cancer. It successfully stratified patients into low-risk and high-risk groups, with the latter developing rapid systemic and local relapses. The pCR nomogram score may be helpful for identifying patients at higher risk of relapse who may benefit from clinical trials of intensified therapy.
Acknowledgements
The work was supported in part by institutional funding from The University of Texas MD Anderson Cancer Center and by Cancer Center Support (Core) Grant CA016672. We thank Christine Wogan, MS, ELS, of MD Anderson’s Division of Radiation Oncology for her expert editing of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Feig BW, Ching DC. eds. The MD Anderson Surgical Oncology Handbook. Philadelphia: Lippincott Williams and Wilkins, 2011.
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [PubMed]
- Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885-91. [PubMed]
- Derogar M, Orsini N, Sadr-Azodi O, et al. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615-9. [PubMed]
- Rohatgi PR, Swisher SG, Correa AM, et al. Failure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagus. Cancer 2005;104:1349-55. [PubMed]
- Rohatgi P, Swisher SG, Correa AM, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer 2005;104:2365-72. [PubMed]
- Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005;23:4330-7. [PubMed]
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. [PubMed]
- Murthy SB, Patnana SV, Xiao L, et al. The standardized uptake value of 18-fluorodeoxyglucose positron emission tomography after chemoradiation and clinical outcome in patients with localized gastroesophageal carcinoma. Oncology 2010;78:316-22. [PubMed]
- Flamen P, Van Cutsem E, Lerut A, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol 2002;13:361-8. [PubMed]
- Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer 2004;101:1776-85. [PubMed]
- Izzo JG, Malhotra U, Wu TT, et al. Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol 2006;24:748-54. [PubMed]
- Luthra R, Wu TT, Luthra MG, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol 2006;24:259-67. [PubMed]
- Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol 2012;23:2638-42. [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [PubMed]
- Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [PubMed]
- Abdel-Latif MM, O’Riordan JM, Ravi N, et al. Activated nuclear factor-kappa B and cytokine profiles in the esophagus parallel tumor regression following neoadjuvant chemoradiotherapy. Dis Esophagus 2005;18:246-52. [PubMed]
- Izzo JG, Wu X, Wu TT, et al. Therapy-induced expression of NF-kappaB portends poor prognosis in patients with localized esophageal cancer undergoing preoperative chemoradiation. Dis Esophagus 2009;22:127-32. [PubMed]
- Luthra R, Wu TT, Luthra MG, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol 2006;24:259-67. [PubMed]
- Monjazeb AM, Riedlinger G, Aklilu M, et al. Outcomes of patients with esophageal cancer staged with [18F]fluorodeoxyglucose positron emission tomography (FDG-PET): can postchemoradiotherapy FDG-PET predict the utility of resection? J Clin Oncol 2010;28:4714-21. [PubMed]
- Chen YM, Pan XF, Tong LJ, et al. Can 18F-fluorodeoxyglucose positron emission tomography predict responses to neoadjuvant therapy in oesophageal cancer patients? A meta-analysis. Nucl Med Commun 2011;32:1005-10. [PubMed]
- De Cobelli F, Giganti F, Orsenigo E, et al. Apparent diffusion coefficient modifications in assessing gastro-oesophageal cancer response to neoadjuvant treatment: comparison with tumour regression grade at histology. Eur Radiol 2013;23:2165-74. [PubMed]
- Weber MA, Bender K, von Gall CC, et al. Assessment of diffusion-weighted MRI and 18F-fluoro-deoxyglucose PET/CT in monitoring early response to neoadjuvant chemotherapy in adenocarcinoma of the esophagogastric junction. J Gastrointestin Liver Dis 2013;22:45-52. [PubMed]


