
Survival outcomes of patients with pathological stage I gastric cancer using the competing risks survival method
Introduction
Gastric cancer plays an important part in cancer burden in Brazil. It represented the fifth most incident malignant neoplasm and the second most common cause of cancer-related death in 2015 (1). Most cases of gastric cancer are diagnosed while the disease is still confined to the stomach and to the regional lymph nodes, and surgery remains the cornerstone of treatment in this setting (2).
However, patients are still at risk of disease relapse even after adequate surgery. In this regard, many landmark trials have established the role of perioperative (3,4) or adjuvant treatments (5-7) in gastric cancer patients. As these studies included almost exclusively patients with stage II or III disease according to the AJCC 8th Edition, very little is known about the role of chemotherapy in patients with less advanced (stage I) gastric cancer.
Before settling the role of chemotherapy in stage I gastric cancer, one needs to estimate survival outcomes of patients treated exclusively with surgery and determine factors associated with increased risk of disease relapse. Current data indicate 5-year overall survival (OS) rates exceeding 90% (8) and a multitude of different prognostic factors have been associated with a worse prognosis in this group of patients, such as presence of perineural or angiolymphatic invasion, larger tumor size, and proximal tumor location (8-10). These factors may potentially patients with stage I gastric cancer for whom perioperative or adjuvant chemotherapy might be beneficial.
At the same time, this analysis is complicated by the fact that studies performed so far in this population have relied solely on naïve estimates of survival, not taking into account the risk of death from causes other than gastric cancer. Indeed, retrospective data support that patients with early gastric cancer are more likely to die of concurrent comorbidities or second primary tumors than of gastric cancer (11). Thus, survival analyses that accommodate competing causes of mortality are preferred to establish the risk of gastric cancer recurrence (and death) and to indicate potential predictors of disease recurrence.
Therefore, we designed a retrospective study to describe the outcomes of patients with pathological stage I gastric cancer treated exclusively with surgery in our institution using the competing risks survival method. We also aimed to evaluate the role of putative harbingers of gastric cancer recurrence, death from gastric cancer and death from causes other than gastric cancer. Also, due to the results of the FLOT4 study (which included patients with clinical T2N0 tumors), we also performed a predefined analysis of outcomes in the subgroup of patients with pathological T2N0 tumors.
Methods
This is a retrospective, analytical, unicentric study performed in a cancer-dedicated hospital. It was approved by the institutional Internal Ethics Review Board.
Patients
We included patients aged 18 years old and above, with pathologically confirmed diagnosis of gastric adenocarcinoma, classified as pathological stage I (IA and IB) according to AJCC 8th Edition, and submitted to upfront surgery between January 1996 and December 2015. Patients diagnosed with other invasive neoplasm within five years of the diagnosis of gastric cancer, those experiencing post-operative death, and those undergoing adjuvant treatment were excluded.
Variables
We collected data about the following patients’ characteristics: age, gender, age-adjusted Charlson comorbidity score (AACCS), ASA (American Society of Anesthesiology) scale, and body mass index. We also gathered the following information about the disease: primary tumor site, histopathological type (according to Lauren’s classification), histopathological grade, pathological T staging, pathological N staging, blood vascular invasion (BVI), lymphovascular invasion (LVI), and perineural invasion (PNI). The following surgical data were retrieved: type of surgery, type of lymphadenectomy, number of resected lymph nodes, surgical complications according to the Clavien-Dindo classification, and surgical margins.
Outcomes
The co-primary outcomes of the study were the cumulative probabilities of death from gastric cancer and from causes other than gastric cancer according to the competing risks survival method (CRSM). As secondary outcomes, we evaluated the cumulative risk of gastric cancer relapse using CRSM, and Disease-free survival (DFS) and OS according to naïve Kaplan-Meier curves. DFS was defined as the time from surgery to gastric cancer recurrence or death from any cause. OS was defined as the time from surgery to death from any cause. We also aimed to name the specific causes of death not resulting from gastric cancer. We performed such analyses on the whole population, as well as on the subgroup of patients with pT2N0 tumors. As an exploratory analysis, we calculated subdistribution hazards (SH) for gastric cancer recurrence, death from gastric cancer, and death from causes other than gastric cancer to evaluate the role of putative harbingers of gastric cancer recurrence, gastric cancer-related death, and death from other causes.
Statistical analysis
Qualitative variables were expressed as absolute frequencies and simple ratios, and quantitative variables were summarized using median values and interquartile ranges (IQR). We estimated naïve DFS and OS curves according to the Kaplan-Meier estimator. Cumulative incidence probabilities curves that comply with competing risks were estimated in a pair-wise fashion for gastric cancer recurrence and death from causes other than gastric cancer, and for death from gastric cancer and death from causes other than gastric cancer. We fitted univariate sub-distribution hazards of an event for different variables according to the Fine-Gray model and generated models using biologically plausible variables in which the P value was <0.25 in the univariate analysis. For this reason, variables regarding pathological characteristics of gastric cancer were excluded from the multivariate model of death from causes other than gastric cancer (12). The significance level of the tests was fixed at 0.05. All statistical analysis was performed using R software version 3.5 (R Core Team, 2018).
Results
We identified 216 patients who met the inclusion criteria. Thirty-one patients were excluded for the following reasons: post-operative death (nine patients), diagnosis of other invasive neoplasm within five years of the diagnosis of gastric cancer (13 patients), and adjuvant treatment (nine patients).
Table 1 describes the clinicopathological characteristics of the study population (n=185) and of the pathological T2N0 subgroup (n=37). In the study population, median age was 61.0 years (IQR, 52.0–71.0 years) and most patients were male (54.6%; n=101). Median AACCS was 4 (IQR, 3.0–5.0) and most patients were classified as ASA 2 (66.8%; n=123). Most tumors were located in the gastric antrum (50.3%; n=93) and the most frequent histopathological type was intestinal-type adenocarcinoma (54.9%; n=100).
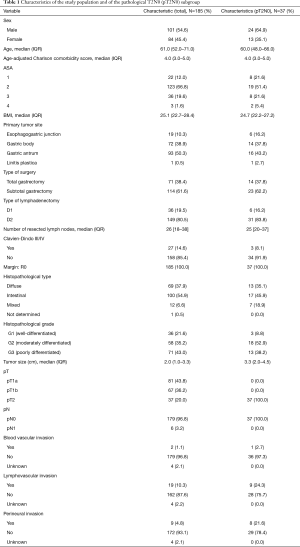
Full table
Within the study population, most patients (61.6%; n=114) were treated with a subtotal gastrectomy and 80.5% (n=149) underwent a D2 lymphadenectomy. The median number of resected lymph nodes was 26 (IQR, 18–38). All patients underwent an R0 resection. pT1a was the most common tumor pathological staging (43.8%; n=81). LVI invasion was found in 10.3% of tumors (n=19) and PNI in 4.8% of tumors (n=9).
Survival outcomes (Figures 1,2)


Median follow-up for the entire population was 75.6 months (95% CI, 66.8–85.2 months) and 27 patients (14.6%) were lost to follow-up. In the study population, 7 patients (3.8%) experienced disease recurrence (Table 2). There were three locoregional and four systemic recurrences. Among patients with pT2N0 (n=37), three patients experienced distant relapse. In the study population, DFS rates at 24 and 60 months were 96.1% and 90.2%, respectively (Figure 1A). In the pT2N0 subgroup, DFS rates at 24 and 60 months were 88.9% and 79.3%, respectively. According to the competing risks analysis, the probabilities of recurrence were 1.6% at 24 months and 3.0% at 60 months in the study population, and 5.5% at 24 months and 8.6% at 60 months in the pT2N0 subgroup (Figure 2A). None of the six patients with pathological T1N1 disease experienced disease relapse.
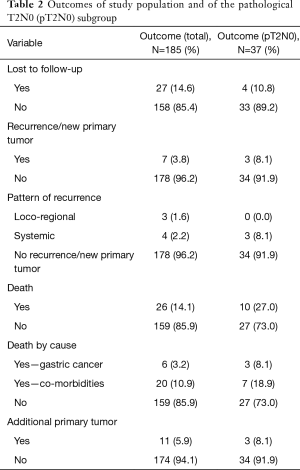
Full table
In the study population, 26 patients were dead at last follow-up. Gastric cancer was the single most important cause of mortality (23.1%; n=6). Nonetheless, other causes of death comprised 76.9% (n=20) of all deaths (Table 3). In the study population, OS rates at 24 and 60 months were 96.6% and 90.7%, respectively (Figure 1B). In the pT2N0 subgroup, OS rates at 24 and 60 months were 88.9% and 79.3%, respectively. According to the competing risks analysis, the probabilities of death from gastric cancer were 1.1% at 24 months and 2.5% at 60 months in the study population. In this group, the probabilities of death from causes other than gastric cancer were 2.2% at 24 months and 6.7% at 60 months. Also, the changes of dying from gastric cancer were 5.5% at 24 months and 8.6% at 60 months in the pT2N0 subgroup. In the same group, the probabilities of death from causes other than gastric cancer were 5.5% at 24 months and 12.0% at 60 months (Figure 2B). Additionally, eleven patients (5.9% of the study population) had the diagnosis of a second primary neoplasm, the most important one being hepatocellular carcinoma (n=3).
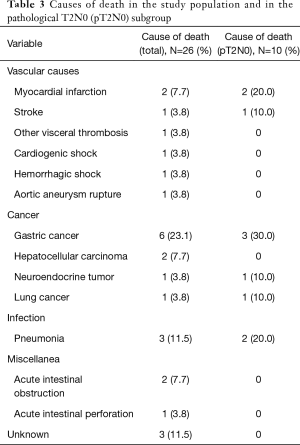
Full table
Prognostic factors
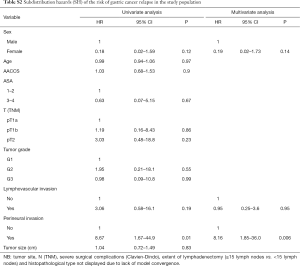
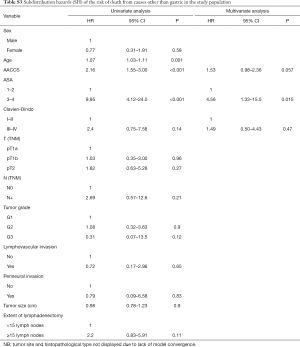
Tables S1-S3 describe predictors of gastric cancer recurrence, death from gastric cancer, and death from causes other than gastric cancer in the study population according to the competing risks survival method. PNI was the only variable independently associated with the risk of gastric cancer relapse (95% CI, 8.16; 95% CI, 1.85–36.0; P=0.006) and of death from gastric cancer (95% CI, 9.67; 95% CI, 2.11–44.3; P=0.004). Regarding the risk of death from causes other than gastric cancer, ASA 3–4 (95% CI, 4.56; 95% CI, 1.33–15.5; P=0.015) and higher AACCS (95% CI, 1.53; 95% CI, 0.98–2.36; P=0.057) were associated with worse prognosis.
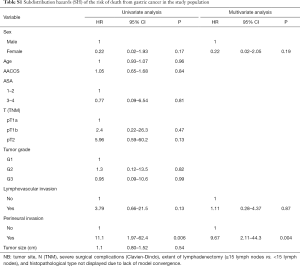
Full table
Full table
Full table
Discussion
Stage I gastric cancer represents roughly 20% of all gastric malignant neoplasms in the West (13). Nonetheless, this subgroup of patients has been systematically underrepresented in randomized trials of adjuvant or perioperative treatment. The only study evaluating the role of chemotherapy in gastric cancer which included patients with pathological stage I disease was the Intergroup 0116 trial (5). In this study, the use of adjuvant chemotherapy plus chemoradiation was associated with improvements in relapse-free and overall survival in patients with stage IB to IV (M0) gastric cancer according to AJCC 3rd Edition. However, patients with stage IB comprised only a small proportion of the study population and there are significant concerns about the quality of the lymphadenectomy in this trial. Also, the AJCC 3rd edition considered as stage IB tumors those that are currently staged as pT3N0 (stage IIA) (14). As a result, the role of multimodality treatment in stage I gastric cancer is largely unknown.
The prognosis of patients with pathological stage I gastric cancer is somewhat worse than that of patients with stage IA disease. Among patients with pathological stage I, those with pT2 gastric cancers fare worse than those with pT1 tumors (15). Also, among patients with disease restricted to the mucosa or the submucosa, the presence of positive lymph nodes is associated with inferior survival (16). Our study corroborates these findings as, despite the lack of statistical significance, there was an increased risk of disease relapse and death from gastric cancer with increasing T stage in the univariate analysis. Thus, the aforementioned arguments make patients with stage IB gastric cancer a more suitable group to study the role of multidisciplinary treatment in early gastric cancer.
Despite the worse prognosis of patients with stage IB gastric cancer (pT2N0 or pT1N1) when compared to stage IA, survival is still fairly good. OS rates at five years ranging from 85% to 90% have been described in retrospective series from Asia (10,17). Nonetheless, more modest figures have been reported in the West (18), especially in studies evaluating nation-wide databases (19). That difference might be explained by the quality of lymphadenectomy, as studies from Asia tend to report higher number of harvested lymph nodes when compared with studies from the West. This subject is so important that according to a retrospective analysis, patients with pT2N0 tumors with inadequate lymphadenectomy (<15 lymph nodes) present poorer survival outcomes and they represent the sole group of patients with pT2N0 gastric cancers in which there seems to be a benefit of adjuvant treatment (19). In our study, most patients (80.5%) underwent a D2 lymphadenectomy and this figure is probably related to the lack of accurate staging methods in the early years of the cohort and to changes in the definition of the extent of the lymphadenectomy with time. That said, we believe surgical quality might explain the relatively good survival outcomes in this group of patients in our institution.
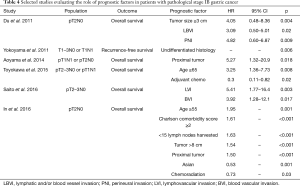
The higher gastric cancer relapse rate found in stage IB disease suggests that patient in this subgroup harbor a rather heterogenous group of tumors. So, to properly select patients with stage IB gastric cancer for multimodality treatment, one might have to identify risk factors for disease recurrence. Many studies have established risk factors for recurrence in patients with stage IB gastric cancer (Table 4). The following features have been associated with increased risk of recurrence or death in patients with stage I gastric cancer: PNI, BVI, LVI, proximal tumor site, undifferentiated histology and tumor size ≥3 cm (8-10,20). In our study, we identified PNI as the only factor independently associated with increased risk of gastric cancer recurrence and death from gastric cancer. We acknowledge that this might reflect the small number of events in this population. Nonetheless, our data corroborate previous findings supporting PNI as the single most important predictor of recurrence in patients with stage IB gastric cancer after surgery (9). Additionally, LVI was also associated with worse oncological outcomes in the univariate analysis. Again, due to the relatively small number of events in our study, one cannot rule out the possibility that LVI and other prognostic factors play a significant part in determining the outcome of patients with stage I gastric cancer.
Full table
The survival outcomes of patients with pathological stage IB gastric cancer treated with adequate lymphadenectomy raises the question if we should offer neoadjuvant chemotherapy for patients with clinical T2N0 gastric cancers. Recently, the FLOT4 trial demonstrated benefits both in terms of overall and DFS for patients undergoing FLOT regimen as compared to ECF or ECX in the perioperative setting (21). Patients with clinical T2N0 were allowed to be enrolled in this trial and subgroup analysis showed that treatment intensification also benefited patients with clinical T2 tumors. Nonetheless, patients with clinical T2 tumors comprise a small proportion of patients in this trial and it remains to be shown how many of such patients actually had positive lymph nodes at staging.
One argument in favor of including patients with clinical T2N0 tumors in trials of perioperative chemotherapy relies on the relatively limited staging accuracy of either CT scan or endoscopic ultrasound (22). However, these results might reflect the use of outdated technologies and recent data demonstrate that, by combining both CT scan and endoscopic ultrasound, gastric cancer staging accuracy can be significantly improved (23). Furthermore, a recent study evaluating patients with clinical T2N0 gastric cancer on a nation-wide database could not identify any benefit of perioperative chemotherapy (24). In this sense, given the good prognosis of most patients with pT2N0 gastric cancer, we would favor an approach in which patients properly staged with CT scan and endoscopic ultrasound as clinical T2N0 would proceed immediately to surgery. Based on the results of our study and on the available information, we believe for those eventually proved to harbor pathologic T2N0 gastric cancer, a discussion regarding the use of adjuvant chemotherapy would rely on quality of the lymphadenectomy and on the presence of risk factors for disease recurrence.
It is also clear from our analysis that most patients with stage I gastric cancer perish from causes other than gastric cancer. We showed that only six deaths among 26 were secondary to gastric cancer. Additionally, nearly 6% of all patients developed second primary tumors. Indeed, second primary tumors and cardiovascular diseases were among the most significant causes of death in this population. This finding is not new (11) and it reinforces the need to focus on the control of comorbidities and on screening strategies in this group of patients.
This find also draws attention to the potential bias of evaluating survival outcomes and the role of putative prognostic factors in patients with stage I gastric cancer using usual survival analysis methods. Both OS and DFS analyses deal with death from any cause as an event. However, to understand the natural history of a neoplasm and to establish the role of prognostic factors in such a disease, one must rule out the events that are biologically non-related to the cancer, such as death from other causes. One way it can be accomplished is through cancer-specific survival. Nevertheless, significant bias still may arise, as in this type of analysis, patients dying from causes other than cancer are censored at the last follow-up. This creates a violation of an important assumption of the Kaplan-Meier method–the assumption of random censoring (25). Thus, in situations in which the risk of an event from competing causes is significant, we believe survival outcomes should be ideally addressed by the use of the competing risks survival model.
Our study is not without limitations. We retrospectively assessed the prognosis of pathological stage I gastric cancer patients in a single cancer-dedicated hospital using routinely collected data. Moreover, in this study we included patients treated for gastric cancer at a time when adequate imaging staging was not available. Therefore, adequate clinical staging is lacking. Also, most of our patients had stage IA gastric cancers and we cannot exclude the possibility that some of the local recurrences were indeed second primary gastric tumors. Additionally, some 15% of all patients were lost to follow-up, and the number of events in our study is modest. We acknowledge that this might hamper the evaluation of putative prognostic factors in early gastric cancer. Nevertheless, out study also has some virtues. The small number of events demonstrate the excellent survival outcomes of patients with stage I gastric cancer submitted to gastrectomy and adequate lymphadenectomy, emphasizing the results of previous Asian studies. Furthermore, our data corroborate the findings of a previous investigation regarding the prognostic role of PNI in stage I gastric cancer. Finally, we believe we have analyzed survival outcomes using a more appropriate statistical model, allowing for competing causes of mortality.
Conclusions
The prognosis of patients with stage IA gastric cancer is excellent, with very few recurrences. Patients with stage IB gastric cancer also fare well, but those harboring tumors with PNI present a higher risk of disease relapse. We believe that should adjuvant chemotherapy be considered in the setting of stage IB gastric cancer, selection of patients based on the risk of relapse is paramount, and in that sense, PNI can be used as a harbinger of disease recurrence. Most patients with early gastric cancer died from unrelated causes. We believe more efforts should be concerted in controlling comorbidities and screening for second primary tumors in this population. Finally, studies to come in this setting should be designed to consider competing causes of mortality, as this approach more accurately represents the oncological outcomes of patients with early stage gastric cancer.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a retrospective, analytical, unicentric study performed in a cancer-dedicated hospital. It was approved by the institutional Internal Ethics Review Board.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2015: Cancer of the Stomach [Internet]. 2018 [cited 1 June 2019]. Available online: https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_24_stomach.pdf
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Saito H, Murakami Y, Miyatani K, et al. Predictive factors for recurrence in T2N0 and T3N0 gastric cancer patients. Langenbecks Arch Surg 2016;401:823-8. [Crossref] [PubMed]
- Du C, Zhou Y, Huang K, et al. Defining a high-risk subgroup of pathological T2N0 gastric cancer by prognostic risk stratification for adjuvant therapy. J Gastrointest Surg 2011;15:2153-8. [Crossref] [PubMed]
- Aoyama T, Yoshikawa T, Fujikawa H, et al. Prognostic factors in stage IB gastric cancer. World J Gastroenterol 2014;20:6580-5. [Crossref] [PubMed]
- Guadagni S, Catarci M, Kinoshita T, et al. Causes of death and recurrence after surgery for early gastric cancer. World J Surg 1997;21:434-9. [Crossref] [PubMed]
- Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 2010;45:1388-95. [Crossref] [PubMed]
- In H, Solsky I, Palis B, et al. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol 2017;24:3683-91.
- Beahrs OH, Henson DE, Hutter RV, et al. Chapter 10: Stomach. In: Lippincott JB. editor. Manual for Staging of Cancer. Third edition. Pennsylvania: Philadelphia, 1988:69-74.
- Ikoma N, Blum M, Chiang YJ, et al. Survival rates in T1 and T2 gastric cancer: A Western report. J Surg Oncol 2016;114:602-6. [Crossref] [PubMed]
- Folli S, Dente M, Dell'Amore D, et al. Early gastric cancer: prognostic factors in 223 patients. Br J Surg 1995;82:952-6. [Crossref] [PubMed]
- Toyokawa T, Ohira M, Sakurai K, et al. The Role of Adjuvant Chemotherapy for Patients with Stage IB Gastric Cancer. Anticancer Res 2015;35:4091-7. [PubMed]
- Hochwald SN, Brennan MF, Klimstra DS, et al. Is lymphadenectomy necessary for early gastric cancer? Ann Surg Oncol 1999;6:664-70. [Crossref] [PubMed]
- In H, Kantor O, Sharpe SM, et al. Adjuvant Therapy Improves Survival for T2N0 Gastric Cancer Patients with Sub-optimal Lymphadenectomy. Ann Surg Oncol 2016;23:1956-62. [Crossref] [PubMed]
- Yokoyama T, Kamada K, Tsurui Y, et al. Clinicopathological analysis for recurrence of stage Ib gastric cancer (according to the second English edition of the Japanese classification of gastric carcinoma). Gastric Cancer 2011;14:372-7. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:abstr 4004.
- Fairweather M, Jajoo K, Sainani N, et al. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol 2015;111:1016-20. [Crossref] [PubMed]
- Li JH, Shen WZ, Gu XQ, et al. Prognostic value of EUS combined with MSCT in predicting the recurrence and metastasis of patients with gastric cancer. Jpn J Clin Oncol 2017;47:487-93. [Crossref] [PubMed]
- Gabriel E, Attwood K, Narayanan S, et al. Does neoadjuvant/perioperative chemotherapy improve overall survival for T2N0 gastric adenocarcinoma? J Surg Oncol 2018;117:659-70. [Crossref] [PubMed]
- Läärä E, Korpi JT, Pitkanen H, et al. Competing risks analysis of cause-specific mortality in patients with oral squamous cell carcinoma. Head Neck 2017;39:56-62. [Crossref] [PubMed]


