
Phase Ib/II study combining tosedostat with capecitabine in patients with advanced pancreatic adenocarcinoma
Introduction
Approximately 55,000 new cases of pancreatic ductal adenocarcinoma (PDAC) are diagnosed annually in the United States. PDAC is projected to be the second leading cause of cancer-related death by 2030 (1). Current standard therapies for metastatic PDAC include FOLFIRINOX (5-FU/LV, irinotecan, oxaliplatin) with median overall survival (mOS) of 11.1 months (2), and gemcitabine/nab-paclitaxel with mOS 8.5 months (3). Given the considerable toxicity of FOLFIRINOX, a large percentage of patients with advanced PDAC receive gemcitabine-based regimens in the front-line setting. Five-FU/leucovorin (LV)/nanoliposomal-irinotecan (nal-IRI) is a second-line treatment option for patients who have progressed on a gemcitabine-based regimen, however median progression-free survival (PFS) is modest at 3.1 months (4). Thus, there is great need for more effective regimens in the second or later lines of therapy.
Aminopeptidases are N-terminus directed peptidases that degrade peptides resulting from proteasomal degradation into free single amino acids, and are over-expressed in multiple malignancies where they provide a continual supply of free amino acids to support anabolic processes and cellular growth. Multiple aminopeptidases are expressed in most cells, including aminopeptidase N (APN), leukotriene A4 (LTA4) hydrolase, puromycin-sensitive aminopeptidase (PuSA) and leucine aminopeptidase (LAP) [reviewed in (5)]. Amino acid starvation or treatment with small molecule aminopeptidase inhibitors leads to depletion of the cellular free amino acid pool and inhibition of the mTOR (mammalian target of rapamycin) pathway, as well as a signature transcriptional response to up-regulate expression of genes involved in de novo amino acid biosynthesis; collectively, this constitutes the amino acid deprivation stress response (AADR) (6).
Notably, aminopeptidase N (APN) is over-expressed in PDAC relative to benign pancreatic tissue, and higher expression levels of APN correlate with inferior overall survival (7,8). The aminopeptidase inhibitor tosedostat (CTI BioPharma, Seattle) has produced encouraging results in relapsed/refractory acute myeloid leukemia (AML) (9), and has been evaluated in a phase I study of advanced solid tumors demonstrating tolerability and preliminary efficacy, with 4 out of 40 patients experiencing stable disease for greater than 6 months, and one with renal cell carcinoma (RCC) experiencing a partial response (10). Given the high frequency of aminopeptidase over-expression in PDAC, and its correlation with poorer outcomes, we conducted a phase Ib/II trial combining tosedostat with capecitabine as a second-line regimen.
Methods
Study design and participants
This was a single center open-label phase Ib/II study in patients with locally advanced or metastatic PDAC to evaluate the safety and efficacy of tosedostat in combination with capecitabine. The study was conducted at Washington University in St. Louis, St. Louis MO, USA. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Washington University in St. Louis. Patients eligible for enrollment were at least 18 years of age, had histologically or cytologically proven metastatic or unresectable PDAC, and had progressed on or were intolerant of prior gemcitabine-based therapy. Inclusion criteria required ECOG performance status of 0–2, and adequate bone marrow and organ function [ANC >1,000/µL, platelets >100,000/µL, total bilirubin ≤2 mg/dL, creatinine ≤2 mg/dL, AST or ALT ≤2.5× ULN (≤5× ULN in the setting of liver metastases)]. Exclusion criteria included prior chemotherapy or radiotherapy within two weeks of study entry, known brain metastases, prior treatment with aminopeptidase inhibitor, known dihydropyrimidine dehydrogenase deficiency (DPD), significant cardiovascular disease or known HIV positivity on combination anti-retroviral therapy. The study protocol was approved by the Washington University Institutional Review Board (IRB #201503074), and all patients provided written informed consent. The study outcomes will not affect the future management of the patients involved.
Procedures
Tosedostat is an oral drug, which was taken on an outpatient basis daily on a 21-day cycle, at approximately the same time every day with food. Dosing of tosedostat during the phase Ib (lead-in phase) followed a 3+3 dose de-escalation design beginning with tosedostat 120 mg oral twice daily (dose level 0) on days 1–21 of each 21-day cycle. Previous studies identified a maximum tolerated dose (MTD) of tosedostat of 320 mg daily, and 240 mg as the maximum accepted dose (10). As there are no significant overlapping toxicities between tosedostat and capecitabine, a dose of tosedostat 120 mg oral twice daily was chosen as the entry dose level. In the event of dose-limiting toxicity (DLT) at dose level 0, tosedostat was to be dose-reduced to 60 mg twice daily (dose level 1); no further dose-reductions were allowed. Capecitabine dosing began at the standard dose of 1,000 mg/m2 twice daily (dose level 0) on days 1–14 of each 21-day cycle. For adverse events that were deemed related to capecitabine, dose reduction to 750 mg/m2 twice daily (dose level 1) and 500 mg/m2 twice daily (dose level 2) were allowed. Dose re-escalation was not allowed. No pre-medications were required for either tosedostat or capecitabine. Toxicity monitoring by complete blood count (CBC) and comprehensive metabolic panel (CMP), and cardiac monitoring by troponin I or T, brain natriuretic peptide (BNP), electrocardiogram (ECG) and echocardiogram were performed on day 1 of each cycle. Tumor assessment by CA19-9 tumor marker was performed on day 1 of each cycle, and computed tomography (CT) scans were performed at the end of each even-numbered cycle. Safety assessments were done in compliance with the Washington University Institutional Data and Safety Monitoring Plan. The study principal investigator and research patient coordinator continuously monitored for serious toxicities on an ongoing basis. Patients were treated until progression of disease or unacceptable toxicity.
Outcomes
The primary endpoint of the phase Ib was the RP2D of the combination therapy. For the phase II portion of the study, the primary endpoint was the PFS of the combination therapy.
Statistical analysis
OS was defined as the months from the date of treatment start to date of death. Alive patients were censored at the last known date of follow up. OS was estimated by the Kaplan-Meier method and the survival probabilities at some time points were provided. Treatment failure free survival (TFFS) duration was defined as the months from the date of treatment start to the date of treatment end; patients without related AE or withdrawal were censored at the date of treatment end. PFS was defined as the months from the date of treatment start to the date of progression; patients without progression were censored at the last known date of follow up. The initial study design planned for 36 patients, with a power of 0.8 to identify an increase of median PFS from 2 to 4 months at 0.05 significance level, assuming 12 months of accrual and 6 months of follow-up.
Results
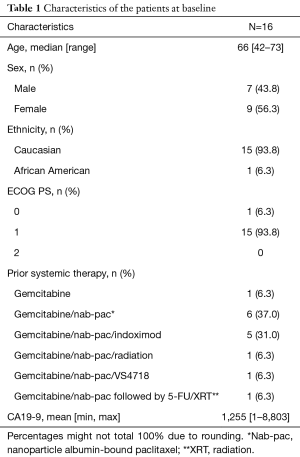
Between October 2015 and December 2017, 16 patients were enrolled on our phase Ib/II study of tosedostat with capecitabine, including six patients in phase Ib (the dose de-escalation phase), and 10 patients in the phase II. The trial was closed to accrual prematurely on 12/20/2017 due to insufficient funding and drug supply from the manufacturer (CTI BioPharma). Patient demographics are presented in Table 1. The median age was 66 years old, with a slight predominance of female patients. The majority of patients had an ECOG performance status of 1. Fourteen patients had metastatic disease, one had locally advanced disease, and the presence of metastases was unknown for one patient. Typical sites of metastases were all represented (liver, omentum, peritoneum, lungs). A total of six patients were treated at the starting dose level of tosedostat 120mg twice daily with capecitabine 1,000 mg/m2 twice daily in the phase Ib portion, and one patient experienced a DLT (grade 3 acute coronary syndrome) (Table 2), thus tosedostat 120mg twice daily was identified as the RP2D. There were a total of five grade 3 treatment-related adverse events observed (acute coronary syndrome, nausea, prolonged QTc interval, diarrhea, fatigue). Among six patients in the phase Ib part, three discontinued treatment due to toxicity [grade 3 fatigue, acute MI prior to assessment of treatment response, and grade 2 decreased ejection fraction (EF)] and three due to disease progression. One of 10 patients in the phase II cohort experienced grade-3 toxicity (QTc prolongation); no grade-4 or -5 toxicities were observed in the phase II cohort (Table 2). No deaths were attributed to tosedostat. The study protocol required monitoring of cardiac EF, mandating holding, dose-reduction or discontinuation of tosedostat for grade 2 or 3 decreases in LV EF. Including the patient in the phase Ib cohort, a total of 5 of 16 patients (31%) came off study due to grade 2 decreased EF, all of which were asymptomatic and self-resolved with holding of tosedostat. The average time to worsening of EF was 65 days, and average time to resolution was 30 days.
Full table

Full table
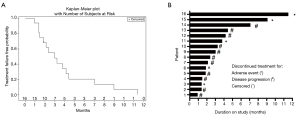
Figure 1 shows treatment failure free survival (TFFS) and duration on study. The median TFFS was 3 months, and eight patients remained on study with stable disease for greater than 3 months. No radiographic partial (PR) or complete (CR) responses were observed. Sixty-two percent of patients (10/16) had a best response of stable disease, and 11/16 evaluable patients discontinued treatment due to disease progression. The median PFS was 7.0 months (Figure 2).
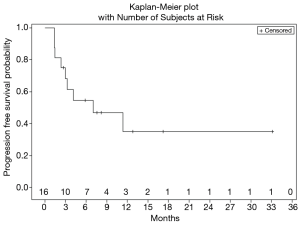
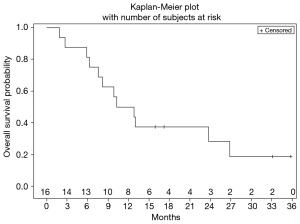
Ultimately, the study was terminated early due to lack of drug availability. At the time of study termination, all enrolled patients had been removed from study due to adverse events or disease progression, however 14/16 (87%) of them remained alive receiving subsequent lines of therapy. mOS was 11.5 months (Figure 3), and median duration of follow-up was 11.5 months.
Discussion
Patients with advanced or metastatic pancreatic cancer face limited effective treatment options. The two most commonly used front-line regimens, gemcitabine/nab-paclitaxel and FOLFIRINOX, yield response rates of 20–30%, and mOS of 8.5 and 11.1 months (2,3). Gemcitabine/nab-paclitaxel is less toxic, thus patients are often treated with front-line gemcitabine-containing regimens. 5-FU-based regimens are often used for patients who progress on front-line gemcitabine therapy, supported by the superior efficacy of 5-FU and oxaliplatin (OFF) over best supportive care (BSC) in this setting (11). However, due to declining performance status, many patients are unable to receive oxaliplatin in the second-line setting, often treated with single-agent 5-FU or capecitabine. Thus, additional non-toxic second-line regimens building on a fluoropyrimidine backbone are needed along with new molecular targets to provide meaningful improvements in outcome.
Previous studies have investigated the aminopeptidase inhibitor tosedostat in elderly patients with relapsed/refractory AML, displaying CR or CR with incomplete platelet recovery (CRi) in 10% of patients (9). The first-in-human phase I study of tosedostat monotherapy in advanced solid tumors demonstrated tolerability and preliminary efficacy in a subset of patients; however only one patient had pancreatic adenocarcinoma, precluding any definitive conclusions regarding its efficacy in this malignancy (10). To our knowledge, ours is the first study to evaluate an aminopeptidase inhibitor in combination with standard chemotherapy in advanced PDAC.
Based on the phase Ib portion of our study, we identified the RP2D of tosedostat as 120mg oral twice daily with capecitabine 1,000 mg/m2 twice daily. The regimen was tolerable, with no grade-4 or grade-5 toxicities, and some grade-3 toxicities. However, there was a high incidence of low-grade asymptomatic decrease in EF, necessitating removal of 5/16 patients from study. Interestingly, three of those affected patients had high-normal baseline EF such that at their nadir, EFs remained in the near low-normal range, with an average EF decrease of 15%. No patients experienced congestive heart failure. Importantly, all occurrences of depressed EF were asymptomatic and self-resolved with holding of therapy. This degree of cardiac toxicity is milder than that observed in a previous report of tosedostat in AML, wherein 3/73 patients (4%) were documented to experience congestive heart failure, where one patient received a daily dose of tosedostat 120 mg and two patients received a daily dose of 240 mg (9). At present, the mechanism of cardiotoxicity due to tosedostat is unknown. Together, these observations suggest caution is advised when treating patients with baseline heart disease, as well as frequent monitoring of patients without known heart disease.
The combination of tosedostat/capecitabine had moderate clinical benefit, with no patients experiencing a complete or partial response. However, the median TFFS was 3 months and median PFS was 7.0 months. These outcomes are encouraging for a second-line therapy, given a median PFS of approximately 2 months in this setting, and they approximate that with standard of care first-line therapy. However, 8/16 patients experienced stable disease for greater than 3 months while on study. Given the median PFS of 3.1 months with nanoliposomal irinotecan/5-FU/leucovorin, and 1.5 months with 5-FU/leucovorin in the second-line setting as reported in the NAPOLI-1 trial (4), this suggests that a subset of patients may be particularly susceptible to aminopeptidase inhibition. However, the enrollment of only 16 out of a planned 36 patients in our study presents a limitation to our data, due to early study closure. Given our small sample size, comparison to other published data is limited; a study with a larger sample size would be needed to confirm our findings. Among the eight patients with prolonged disease control on our study (greater than 3 months), two patients had an initial biochemical partial response (greater than 20% decrease of CA19-9 from baseline). Notably, those patients with prolonged disease control had variable durations of disease control on front-line chemotherapy, and three of them had amongst the highest baseline CA19-9 values, suggesting that benefit from the regimen is not an artifact of lower disease burden or general chemotherapy sensitivity; perhaps they are uniquely sensitive to amino acid deprivation due to unknown underlying molecular features.
In conclusion, tosedostat in combination with capecitabine as a second-line treatment had an acceptable safety profile and displayed signs of efficacy in a subset of patients with advanced or metastatic PDAC. However, these conclusions are limited, as enrollment was less than 45% (16/36) of patients planned. Toxicities were manageable and did not preclude patients from pursuing subsequent lines of therapy, as 68% went on to further systemic therapy. These data suggest unique molecular susceptibilities to aminopeptidase inhibition in a subset of patients, and warrants further basic science and clinical investigation.
Acknowledgments
This work was supported by CTI BioPharma.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Washington University in St. Louis.
References
- Available online: https://cancer.org
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- Krige D, Needham LA, Bawden LJ, et al. CHR-2797: an antiproliferative aminopeptidase inhibitor that leads to amino acid deprivation in human leukemic cells. Cancer Res 2008;68:6669-79. [Crossref] [PubMed]
- Tang X, Keenan MM, Wu J, et al. Comprehensive profiling of amino acid response uncovers unique methionine-deprived response dependent on intact creatine biosynthesis. PLoS Genet 2015;11:e1005158. [Crossref] [PubMed]
- Ikeda N, Nakajima Y, Tokuhara T, et al. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin Cancer Res 2003;9:1503-8. [PubMed]
- Pang L, Zhang N, Xia Y, et al. Serum APN/CD13 as a novel diagnostic and prognostic biomarker of pancreatic cancer. Oncotarget 2016;7:77854-64. [Crossref] [PubMed]
- Cortes J, Feldman E, Yee K, et al. Two dosing regimens of tosedostat in elderly patients with relapsed or refractory acute myeloid leukaemia (OPAL): a randomised open-label phase 2 study. Lancet Oncol 2013;14:354-62. [Crossref] [PubMed]
- Reid AH, Protheroe A, Attard G, et al. A first-in-man phase i and pharmacokinetic study on CHR-2797 (Tosedostat), an inhibitor of M1 aminopeptidases, in patients with advanced solid tumors. Clin Cancer Res 2009;15:4978-85. [Crossref] [PubMed]
- Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. [Crossref] [PubMed]


