Concurrent chemoradiotherapy with protracted infusion of 5-fluorouracil (5-FU) and cisplatin for locally advanced resectable esophageal cancer
Introduction
Esophageal cancer (EC) is an aggressive and lethal malignancy. In the United States (US), 15,070 patients died from EC last year and the incidence is increasing worldwide (1). Advances in the treatment of patients with EC, which accounts for more than 400,000 deaths a year worldwide (1), have been slow compared to other malignancies.
Surgical resection and definitive chemotherapy and radiation as a single modality treatment for EC produce poor long-term survival which prompts the evaluation of neoadjuvant therapy in the form of chemotherapy and/or radiation. Several clinical trials have explored the optimal neoadjuvant therapy paradigm; however, stage migration and the temporal variation in incidence between the two major histological types of EC make interpreting and applying these trials to clinical practice a daunting task.
The poor long-term outcome associated with surgery alone and the high locoregional recurrence with definitive chemoradiation provided the rational for evaluating neoadjuvant chemoradiation followed by surgery in patients with potentially resectable EC.
To evaluate the role of neoadjuvant chemoradiation followed by surgery in patients with potentially resectable EC, several randomized trials have compared neoadjuvant chemoradiation followed by surgery to other treatment modalities. The majority of these studies were criticized for being underpowered; the Irish trial reported by Walsh et al. randomized 113 patients to 40 Gy radiation in 3 weeks concurrently with cisplatin and 5-fluorouracil (5-FU) followed by surgery versus surgery alone (2). The trial resulted in significant survival improvement in survival; however, it was highly criticized for the low survival in the surgery only arm. A US study by the Cancer and Leukemia Group B (CALGB) intended to randomize 475 EC patients to neoadjuvant chemoradiotherapy with 50.4 Gy over 5.5 weeks concurrent with cisplatin and 5-FU and surgery versus surgery alone. The trial was closed prematurely. In the 56 randomized patients (3), median survival was 4.5 years for chemoradiation patients compared to1.8 years for surgery only patients. More recently, the CROSS trial (4) randomized patients with resectable EC to receive surgery alone or to receive weekly carboplatin and paclitaxel for 5 weeks concurrently with radiation followed by surgery. Median overall survival (OS) was 49.4 months in the chemoradiotherapy-surgery group versus 24.0 months in the surgery group (P=0.003).
To date, several chemotherapy combinations have been used concurrently with radiation therapy for the neoadjuvant treatment of EC with the combination of cisplatin and 5-FU being the most common. Most clinicians use two courses of chemotherapy in weeks 1 and 5 (5-FU continuous infusion for 5 days, and cisplatin 75 mg/m2 for 2 doses at the beginning and the end of treatment). Tepper et al. (3) used cisplatin 100 mg/m2 and 5-FU 1,000 mg/m2/day for 4 days on weeks 1 and 5 concurrent with radiation therapy, while in the University of Michigan study (5), patients received cisplatin 20 mg/m2/day on days 1 through 5 and 17 through 21, 5-FU 300 mg/m2/day on days 1 through 21, and vinblastine 1 mg/m2/day on days 1 through 4 and 17 through 20. In older trials (6), patients received four courses of combined 5-FU (1,000 mg per square meter of body-surface area daily for 4 days) and cisplatin (75 mg per square meter on the first day).
The combined chemotherapy and radiation in the above mentioned trials is, as expected, more toxic than surgery or radiation treatment. Herskovic et al. (6) reported one death related to treatment and severe side effects reported in 44% of patients with 20% life threatening events. In the Irish trial (2), 10% of patients treated with combined therapy had grade 3 toxicity; two patients had grade 4 toxicity and one patient died during treatment. In the Michigan study (5), 78%, 39%, and 31% of patients experienced grade ¾ neutropenia, neutropenic fever and thrombocytopenia respectively and 63% of patients required feeding tubes. In the CALGB 9781 trial (3), 57% of patients receiving preoperative therapy experienced grade 3 or greater hematological toxicity and 42, 34, 24 and 4 percent experienced esophagitis, infection, pain and treatment-related death respectively. Complete pathological response in these studies ranged from 25-40% and OS rate was between 10-39%.
At our institution, for the last 15 years, and in order to increase compliance and decrease toxicity, we adopted a regimen consisting of continuous infusion low-dose 5-FU combined with 2 doses of cisplatin concurrently with radiation as neoadjuvant treatment for patients with potentially resectable EC. Patients presenting at our institute with locally advanced (T3-T4) and/or lymph node (LN)-positive EC who were potentially eligible for surgery have been considered for cisplatin-based combination chemotherapy plus radiation. We analyzed the outcome of all patients that were treated with this regimen since 1997.
Patients and methods
Patients
Patients with potentially resectable EC (>T2) and/or LN-positive disease (≥N1), who were treated with cisplatin/5-FU combination concurrently with radiation, and were scheduled for surgery between 1997 and 2012, were identified from an institutional review board (IRB) approved institutional EC database. These patients were discussed in multidisciplinary fashion with representatives from the departments of surgery, medical oncology, pathology, radiation oncology and radiology. All patients were analyzed for survival and toxicity including patients who did not have their planned resection due to poor condition, metastatic or unresectable disease or refusal. Patients who refused surgery following neoadjuvant therapy went on to receive either more chemotherapy or other modality or they were simply placed on surveillance.
Staging
Staging was done according to the American Joint Committee on Cancer (AJCC) 6th edition, with clinical staging pre- and post-chemotherapy based on thoracic/abdominal and pelvis computer tomography (CT) scans, positron emission tomography (PET) scans, endoscopic ultrasound (EUS) and pathological staging after surgery. Prior to treatment, LN status was confirmed either by imaging/EUS only or in combination with fine-needle aspiration (FNA). All pathology specimens from the initial endoscopic biopsies were read and confirmed by pathologists with specialization in gastrointestinal malignancies. All operations were performed with curative intent and included removal of the primary tumor en bloc with its draining LN. Surgical approaches to esophagectomy included transthoracic, thoracoabdominal, and transabdominal techniques. Generally, the surgery was performed within 12 weeks after the final course of radiation.
Patients were seen and examined every 3 months for the first 2 years, then at every 6 months for years 2-5, and then annually. Routine follow-up exams included, physical exam, history, CT scans of chest/abdomen and pelvis. Endoscopy was performed if clinically indicated.
Chemotherapy and radiation
Chemotherapy
Chemotherapy consisted of a cisplatin-based regimen. Patients who were considered unfit secondary to impaired renal function, co morbidity or low performance status received other regimens (Data not shown). A small number of patients received carboplatin/paclitaxel concurrently with radiation after the publication of the CROSS trial (4).
All patients received cisplatin 75 mg/m2 on days 1 and 29. They received 5-FU continuous infusion 225 mg/m2/day Monday through Friday (Figure 1).
Evaluation of clinical response to therapy was performed by imaging 6-8 weeks following treatment and included CT scans and/or PET scans. Patients with no evidence of metastatic disease and good performance status were referred for surgical resection.
Radiation
Radiation therapy treatment technique was delivered at the discretion of the radiation oncologist; CT-based planning was performed with the patients lying supine with arms up on a Vac-Lock (Civco Medical Solutions, Kalona, IA) immobilization device. Four dimensional (4D) CT simulation scans were obtained to assess tumor motion by respiration.
A clinical target volume (CTV) encompassing a 3-4 cm superior margin, 3-4 cm distal margin, and 3-5 mm radial margin was contoured. For upper thoracic tumors, bilateral supraclavicular lymphatics were included. For distal esophageal and gastroesophageal cancers, celiac nodes and nodes along the left gastric artery were always included in the CTV. For gastroesophageal junction adenocarcinomas, other regional abdominal nodal groups were included based on the Siewert classification.
Surgery
All patients underwent restaging with PET-CT scans 6-8 weeks following chemotherapy and radiation. Patients who were without evidence of metastatic disease and who were deemed medically operable underwent either transhiatal or transthoracic esophagectomy using either open versus laparoscopic versus robotic esophagectomy at the discretion of the surgeon. Patients who underwent surgery were included in the pathological complete response (pCR) analysis. Patient was considered to have pCR if he had no vital residual tumor cells in the surgical specimen.
Follow-up
Patients were seen at a minimum once a week during treatment and followed after treatment according to the National Comprehensive Cancer Network (NCCN) guidelines. Toxicity was graded based on Common Terminology Criteria for Adverse Events (CTCAE), version 4. Acute toxicities were considered if they occurred during or shortly after chemotherapy and radiation.
Data collection and statistical analysis
For this study, our IRB approved comprehensive EC database was queried according to our inclusion criteria for all patients who received cisplatin and 5-FU concurrently with radiation. A total of 129 patients out of 709 patients were determined to be eligible for the analysis.
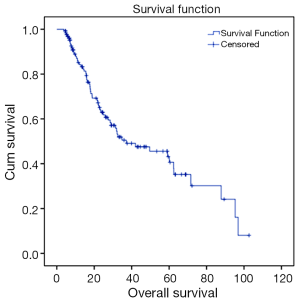
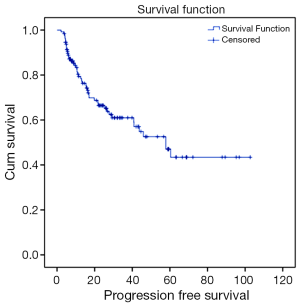
Recurrence rates, recurrence free survival (RFS) and OS were analyzed using the Kaplan-Meier method. OS was defined as the time from diagnosis to any cause of death: patients who were alive at the end of follow-up were censored at that date. Recurrence was defined as first relapse of disease, either loco-regional or distant. RFS was defined as the time from diagnosis until first recurrence or death.
Results
Demographic data
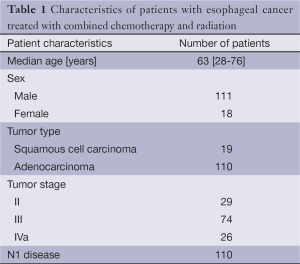
Between July 1997 and June 2012, a total of 129 patients were retrieved from an institutional EC database of 709 patients. Median age of patients was 63 years (range, 28-76 years). Fourteen percent of patients were female and 85% had adenocarcinoma. Twenty-nine, seventy-four and twenty-six patients had stage II, III and IVa disease respectively. One hundred and ten patients had N1 disease based on the AJCC 6th edition. Patient characteristics are shown in Table 1. All patients completed treatment.
Full table
Toxicities
In general, patients tolerated the concurrent chemotherapy and radiation well. All patients completed treatment. Even during hospitalization, patients continued to, at least, receive radiation therapy if clinically appropriate. Less than 10% of patients required dose reduction (25% of the total dose). Fourteen percent of patients had ≥ grade 3 toxicity including constipation, chest pain, confusion, neutropenia in one patient each and anorexia in 18 patients requiring esophageal dilation and/or feeding tube placement +/- esophageal stent. Overall, 18 patients required hospital admission mostly for failure to thrive. Oral mucositis, diarrhea and neuropathy were rarely seen and were mostly grade 1. Treatment related toxicity is summarized in Table 2.

Full table
Outcome
Out of 129 patients, 83 (64%) patients underwent surgical resection between 37 and 149 days following concurrent chemoradiotherapy (CCRT) (median 62 days). R0 resection was achieved in 96% of patients. A pCR was achieved in 38 of 83 patients (46%) who underwent surgical resection. For the entire population, and with a median follow up of 26 months (range, 1.2-144 months); 37% of patients recurred, 38 patients recurred distally and 10 recurred locally. A total of 46% of patients died (Figures 1,2).
Discussion
Concurrent neoadjuvant chemotherapy and radiation in the management of potentially resectable T3-4 and/or LN-positive EC is the most accepted standard of care. Neoadjuvant therapy leads to better delivery of the drugs in untreated, well-vascularized tumors and helps in eradication of the micrometastases. The optimal chemotherapy to be used in this patient population is not known. Our data supports that neoadjuvant protracted infusion of 5-FU plus cisplatin concurrently with radiation therapy is feasible and efficacious in patients with potentially resectable EC. Our patients were able to receive all planned chemotherapy and radiation, allowing for optimal potential benefits of both treatments. Tolerability was reflected in the completion rates of the prescribed treatment. We report the highest pCR in patients who underwent surgical resection and comparable or even superior R0 resection rate and OS.
Several phase II and phase III trials have explored the role of two- or three-drug combination in the neoadjuvant therapy of EC. In these studies, different response rates and complete responses rates have been reported. No randomized trials have been conducted to directly compare chemotherapy regimens, and the optimal combination in this setting remains undefined. The recently published phase III CROSS trial showed significant improvement in outcomes with neoadjuvant therapy compared to surgery alone; however, the dose of radiation used in that trial is lower than the dose recommended by NCCN consensus guidelines. Whether cisplatin/5-FU combination is superior to carboplatin/paclitaxel combination and the optimal doses of chemotherapy and radiation is yet to be established.
In our series, out of the 129 treated patients, all were eligible for survival and toxicity evaluation. Forty-five percent of patients who underwent surgical resection had a pCR which is comparable to the published literature. Grade 3 and 4 toxicities were uncommon. The low number of patients undergoing surgical resection in this series can be explained, at least in part, by the fact that it has been only recently that data has shown improved outcomes with incorporation of surgery. This single institutional data shows the feasibility of the use of neoadjuvant cisplatin and protracted 5-FU in the treatment of EC. Our study has several limitations given its retrospective nature; however, it is encouraging that our results are comparable with previously published data.
Goals of future studies should be to define the most active and safe chemotherapy regimens and radiation modalities. Selecting patients who will most likely benefit from platinum, taxanes or other agents is important and incorporating targeted therapy in adequately powered, randomized trials is necessary. Biomarker driven trials and correlation with treatment outcomes is crucial to identify patients most likely to benefit from personalized treatment approach.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [PubMed]
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. [PubMed]


