
Obesity and younger versus older onset colorectal cancer in the United States, 1998–2017
Introduction
Globally, colorectal cancer (CRC) is the third most deadly and fourth most commonly diagnosed cancer (1). Despite a decrease in CRC among adults 50 and older, incidence and mortality of early onset CRC (EOCRC) in younger adults have steadily increased over the past several decades. Approximately 1 in 10 cases of CRC are diagnosed in individuals under the age of 50 (2,3) and their incidence rates have increased from 8.6 per 100,000 in 1992 to 12.5 per 100,000 in 2015 (3,4). Experts predict that by 2030, the respective incidences of colon and rectal cancers will grow by 90.0% and 124.2% for patients ages 20 to 34, and by 27.7% and 46.0% for patients ages 35 to 49 (3). The etiologies of these trends and whether EOCRC represents a distinct molecular entity remain areas of active investigation.
Known risk factors of CRC include sedentary lifestyle, obesity, diet, alcohol use and smoking, among others (1,5). Although the rising incidence in EOCRC may also be attributed in part to such environmental and lifestyle factors (6,7), there is a paucity of research assessing the association between obesity and EOCRC. As such, we sought to assess whether obesity was associated with prevalence of CRC in younger versus older adults in a nationally representative cohort.
Methods
The National Health Interview Survey (NHIS) is a cross-sectional household survey of noninstitutionalized civilian adults living in the United States assessing a wide range of health status and utilization measures (8). Sample weights are provided for each individual permitting inferences on national prevalence. Harmonized data of participants from 1998–2017 was obtained through the Integrated Health Interview Series (9). Participants are first asked: “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” For those who responded yes, they were subsequently queried, “What kind of cancer was it?” They were also asked at what age they were diagnosed with each cancer. Multivariable logistic regression defined adjusted odds ratios (AORs) and associated 95% confidence intervals (CIs) for self-reporting a diagnosis colorectal cancer. In addition to body mass index (BMI, < vs. ≥30 kg/m2) and age (<50 vs. ≥50 years), relevant sociodemographic variables and potential confounders included in the model were sex, race, ethnicity, insurance status and smoking. Body mass index was dichotomized at 30 which is the cut-off for obesity and younger adults was defined as age younger than 50 based on epidemiologic data showing rising incidence of CRC among this age group (2-4). The analyses were repeated in a separate model including an age (< vs. ≥50 years) *BMI (< vs. ≥30.0 kg/m2) interaction term. Colorectal cancer was defined as self-reporting a diagnosis of colon or rectal cancer and included EOCRC (participants aged 18–49 years reporting history of CRC) and older adult CRC (participants aged ≥50 reporting history of CRC diagnosed at age ≥50). Sample weighting stratified by year defined nationally representative estimates. Statistical testing was 2-sided, with α=0.05. Analyses were performed with Stata/SE 15.1 (StataCorp). The University of Texas Southwestern Medical Center deemed the study to be exempt from review.
Results
Among 583,511 study participants 55.2% (n=321,975) were aged 18–49 years, and there were a total of 3,173 CRC cases (0.5% of cohort) [239 in younger adults (7.5%) and 2,934 (92.5%) in older adults] (Table 1). On multivariable analysis for the entire cohort accounting for sex, race, ethnicity, insurance status, smoking and BMI, the following variables were associated with greater odds of self-reporting a diagnosis of CRC: age ≥50 (AOR 11.06, 95% CI: 9.40–13.02), non-Hispanic ethnicity (AOR 1.77, 95% CI: 1.46–2.15) and cigarette smoking (AOR 1.33, 95% CI: 1.22–1.46) were positively associated with self-reporting a diagnosis of CRC; there was no association between BMI ≥30.0 kg/m2 and diagnosis of CRC (AOR 0.97, 95% CI: 0.89–1.07) (Table 2).

Full table
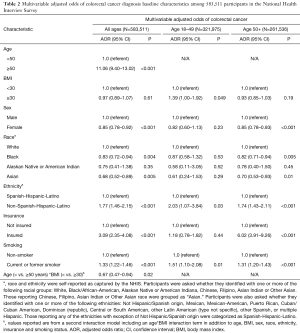
Full table
In the second model, there was a statistically significant age (< vs. ≥50 years) *BMI (< vs. ≥30.0 kg/m2) interaction term (P=0.02) such that among participants aged 18–49 years, BMI ≥30.0 kg/m2 was associated with greater odds of self-reporting a diagnosis of CRC compared to patients with BMI <30.0 kg/m2 (34.1% vs. 27.4%, AOR 1.39, 95% CI: 1.00–1.92) (i.e., among younger adults with history of CRC, 34.1% had BMI ≥30 kg/m2). This interaction was not observed among participants aged ≥50 years (29.6% vs. 31.4%, AOR 0.93, 95% CI: 0.85–1.03) (Table 2). In addition to participants being obese (as defined by the BMI ≥30), non-Hispanic ethnicity (AOR 2.03, 95% CI: 1.07–3.84) and smoking (AOR 1.51, 95% CI: 1.10–2.08) were associated with CRC among younger adults (Table 2).
Discussion
In this nationally representative study spanning 20 years, obesity was associated with CRC in younger but not in older adults. A recent analysis showed that those with higher current BMI were more likely to have EOCRC (10), however this study was limited to predominantly white female nurses and small number of EOCRC cases (n=114). Another single institution study found no difference in rates of obesity between EOCRC and CRC, although their findings may be limited by sample size (11). Multiple other studies have demonstrated associations between obese and overweight BMI, weight gain during adulthood and accumulation of abdominal fat and subsequent development of CRC at any age (18+), without distinguishing between EOCRC and CRC diagnosed in older adulthood (12-15). Thus, to the best of our knowledge, this is the first large study conducted in the modern era among the general population demonstrating an effect of age on obesity and CRC incidence. While substantial evidence has shown that obesity plays a role in the pathogenesis of CRC (1,5) our work suggests this association may be stronger for EOCRC.
A recent review examined the potential mechanisms explaining the association between obesity and CRC (1). In particular, obesity is associated with physical inactivity and may lead to alteration in gut flora along with irritation and inflammation of intestinal epithelium, which could promote carcinogenesis (1). Furthermore, excess body weight could be associated with greater release of free oxygen radicals. Given that obesity is a potentially modifiable risk factor for patients, targeted interventions addressing weight could potentially reduce the incidence of CRC, particularly among younger patients.
Limitations of the study include self-reporting of cancer diagnosis and use of BMI at time of survey rather than prior to diagnosis. However since any bias in correlation between current and pre-treatment weight is likely to be similar for those with CRC at age ≥50 years, the results indicate that obesity could be more important for EOCRC. In addition, participants aged ≥50 years diagnosed with CRC prior to age 50 were not included as having younger adult CRC to permit assessment of effect modification by age subgroup and to exclude those with remote history of CRC for whom current BMI may be least representative of BMI at diagnosis. Other notable limitations include the cross-sectional study design given the evidence of a cohort effect in rise of EOCRC. In addition, there was a strong association between insured status and CRC, likely due to undiagnosed cases among uninsured participants. In addition, racial and ethnic minorities had lower odds of self-reporting a diagnosis of CRC, which could be due to undiagnosed cases among these populations or survivorship bias (i.e., minorities are less likely to survive their diagnosis and participate in the survey). The database also lacks cancer-specific information including stage of diagnosis, treatment regimen, and outcomes, however nationwide databases including these details generally do not include patient BMI. Given the limitations of our study, our findings should be viewed as hypothesis generating and an impetus for additional research examining risk factors including patient weight across a lifetime.
In summary, CRC diagnosis appears to be more common in younger individuals (aged 18–49 years) with BMI ≥30, suggesting an important role of weight control in early adulthood, among other healthy lifestyle behaviors, as a potential risk reduction strategies for CRC. Given the overall prevalence of obesity of 1/3 in participants reporting history of EOCRC, further studies should also address whether obese and overweight BMI contributes to worse treatment toxicity, cancer-specific outcomes and survival. Given that obesity is a modifiable risk factor, lifestyle interventions including diet and exercise could help to address the rising incidence of EOCRC.
Acknowledgments
BA Mahal reports funding from the American Society of Radiation Oncology (ASTRO) and the Prostate Cancer Foundation (PCF).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 2019;14:89-103. [Crossref] [PubMed]
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017. [Crossref] [PubMed]
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150:17-22. [Crossref] [PubMed]
- Murphy CC, Wallace K, Sandler RS, Baron JA. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2019;156:958-965. [Crossref] [PubMed]
- Karahalios A, Simpson JA, Baglietto L, et al. Change in weight and waist circumference and risk of colorectal cancer: results from the Melbourne Collaborative Cohort Study. BMC Cancer 2016;16:157. [Crossref] [PubMed]
- Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18:e457-e471. [Crossref] [PubMed]
- Park SY, Boushey CJ, Wilkens LR, et al. High-Quality Diets Associate With Reduced Risk of Colorectal Cancer: Analyses of Diet Quality Indexes in the Multiethnic Cohort. Gastroenterology 2017;153:386-394.e2. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. National Health Interview Survey: Methods. Available online: http://www.cdc.gov/nchs/nhis/methods.htm, accessed July 1, 2018.
- Lynn A. Blewett, Julia A. Rivera Drew, Risa Griffin, Miriam L. King, and Kari C.W. Williams. IPUMS Health Surveys: National Health Interview Survey, Version 6.3 [dataset]. Minneapolis, MN: IPUMS, 2018. http://doi.org/. [Crossref]
- Liu PH, Wu K, Ng K, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019;5:37-44. [Crossref] [PubMed]
- Gausman V, Dornblaser D, Anand S, et al. Risk Factors Associated With Early-onset Colorectal Cancer. Clin Gastroenterol Hepatol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Celind J, Ohlsson C, Bygdell M, et al. Childhood Body Mass Index Is Associated with Risk of Adult Colon Cancer in Men: An Association Modulated by Pubertal Change in Body Mass Index. Cancer Epidemiol Biomarkers Prev 2019;28:974-9. [Crossref] [PubMed]
- Sandhu MS, Luben R, Day NE, et al. Self-reported birth weight and subsequent risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2002;11:935-8. [PubMed]
- Song M, Hu FB, Spiegelman D, et al. Adulthood Weight Change and Risk of Colorectal Cancer in the Nurses' Health Study and Health Professionals Follow-up Study. Cancer Prev Res (Phila) 2015;8:620-7. [Crossref] [PubMed]
- Song M, Hu FB, Spiegelman D, et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study. Int J Epidemiol 2016;45:871-83. [Crossref] [PubMed]


