Clinical factors associated with the development of postoperative atrial fibrillation in esophageal cancer patients receiving multimodality therapy before surgery
Introduction
The incidence of esophageal cancer (EC) has been increasing in the United States and is associated with a poor prognosis in patients with locally advanced or metastatic disease (1,2). Neoadjuvant therapy consisting of chemoradiation for locally advanced cancers followed by surgical resection is the standard of care (3). However, there are still considerable risks of morbidity and mortality after an esophagectomy (4). Major morbidities associated with esophagectomy include pulmonary complications, anastomotic complications, wound infection, and dysrhythmia (5-7). As one of the most common cardiac complications post-esophagectomy, postoperative atrial fibrillation (POAF) has a reported incidence of 8–22% (6-10). If left unmanaged, POAF can increase the risk of ischemic stroke and myocardial infarction (11-14). Because POAF is often associated with an increased length of hospital stay (LOS) (15,16), we hypothesized that POAF might be a predictable complication of esophagectomy. In this study, we sought to investigate whether POAF was associated with any clinical factors involved with an esophagectomy.
Methods
Patients and treatment
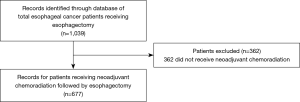
Query of an IRB approved database of 1,039 esophagectomies at Moffitt Cancer Center, Tampa, FL revealed 677 patients with EC from 1999 to 2017 who underwent esophagectomy after neoadjuvant chemoradiation treatment were included in this study (Figure 1). Patients who met this eligibility criteria were treated with chemotherapy consisting of 5-FU based treatment with either cisplatin or oxaliplatin or carboplatin/paclitaxel and radiation with either 2D, 3D, or intensity modulated radiation therapy (IMRT), followed by esophagectomy. Esophagectomy consisted of either Ivor Lewis (transthoracic), transhiatal, three-field and their respective minimally invasive surgery (MIS) methods.
POAF was defined as newly developed AF after esophagectomy prior to discharge that required therapy irrespective of the AF duration. All AF incidents involved a cardiology consultation and management was individualized based on clinical condition and risk factors. Management for rate control included calcium channel blockers and rhythm control with amiodarone. Use of anticoagulation was determined using CHADS risk stratification. Our postoperative protocol is to keep potassium between 4.0–4.5 mEq/L, magnesium above 2.0 mEq/L, and phosphorus near 3.0 mEq/L. Most patients received epidurals for pain management after surgery. If pain was not well controlled, they received patient-controlled analgesia with narcotics. Patients eventually transitioned to oral pain medications primarily oral narcotics and acetaminophen as tolerated.
Statistical analysis
Patient characteristics were evaluated using Pearson Chi-square analysis. Significant dependent variables were then further analyzed using binomial logistic regression. Age, treatment location (primary vs. other), gender, neoadjuvant radiation type (2D vs. 3D vs. IMRT), radiation dose, surgery type (Ivor Lewis vs. transhiatal vs. three field esophagectomy both open and MIS), smoking history, coronary artery disease (CAD), and chronic obstructive pulmonary disease (COPD), operative time, requirement of blood transfusions, volume of fluid management were analyzed when available in the database, onset of POAF, and length of stay (LOS) were analyzed in relationship to the development of POAF with univariate analysis, with statistical significance determined at P<0.05. Clinical factors including age, onset of POAF, and LOS were reported with median values and range. Factors including operative time and volume of fluid administered were reported with mean values and range. Values significant with univariate analysis were then run on multivariate analysis. Statistical analysis was completed using SPSS 24 (Windows Version 24.0; IBM Corp., Armonk, NY, USA).
Results
Patient demographics and disease characteristics
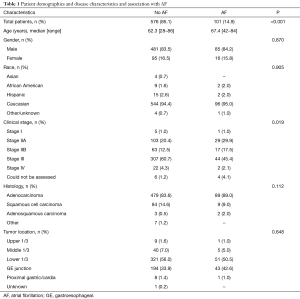
Patient demographics and disease characteristics of the 677 patients in our study are displayed in Table 1. The median age of the entire cohort was 64.3 years (range, 28–86 years), with a white (n=640, 94.6%) male preponderance (n=566, 83.6%). The majority of EC were adenocarcinomas (84.4%) and were stage III at diagnosis (58.2%). Of the entire cohort, 14.9% (n=101) of patients experienced POAF. Increasing age (P<0.001) and clinical stage (P=0.019) was significantly associated with POAF, while gender was not (P=0.870).
Full table
Patient comorbidities and Charlson comorbidity index (CCI)
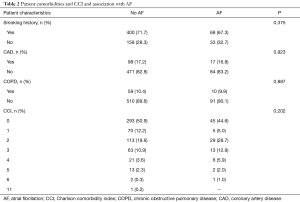
Patient comorbidities and CCI are displayed in Table 2. Although most patients had a smoking history (n=468, 71.0%), only 17.3% (n=116) of the total cohort had CAD and 10.3% (n=69) had COPD. Around half of the patients (n=339, 50.1%) evaluated for esophagectomy did not have major pre-existing comorbidities. Smoking history (P=0.375), CAD (P=0.923), COPD (P=0.887), and CCI (P=0.202) were not associated with onset of POAF.
Full table
Patient treatment location and modality
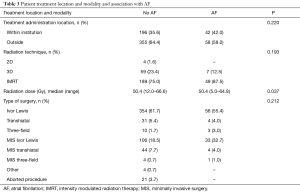
Patient treatment location and modality are displayed in Table 3. Within the entire cohort, over a third (35.6%) of patients undergoing esophagectomy received complete treatment at our institution while the majority (63.8%) of patients received a portion of their cancer treatment elsewhere. Of those receiving radiation as part of their neoadjuvant therapy, 77.3% (n=238) of patients received IMRT with a median dose of 50.4 Gy (range, 12.0–66.6 Gy). Ivor Lewis esophagectomy (81.3%) was the most common type of surgery performed in the entire cohort, followed by transhiatal (12.3%) and three-field (2.7%). Although neoadjuvant radiation dose was significantly associated with POAF (P=0.037), treatment location (P=0.220), radiation technique (P=0.193), and type of surgery (P=0.212) were not.
Full table
Patient operative and post-operative course
Patient operative and post-operative course is displayed in Table 4. Of the patients with available data, POAF occurred most commonly on postoperative day 2. More patients with POAF required blood transfusions during their operative and postoperative course (19.2% vs. 10.6%, P=0.027). Operative time was also longer for patients who developed POAF (368 vs. 328 minutes, P=0.001). Although there was a trend for increased total fluid volume given in the first 24 hours in patients with POAF compared to those who did not (3.4 vs. 3.2 L), there were no statistical differences (P=0.605). There was a statistical difference in length of stay for patients with POAF was 10.5 days (range, 9–17 days) compared to 10.0 days (range, 9–13 days) for those who didn’t (P=0.001).

Full table
Multivariate analysis
In multivariable analysis shown in Table 5, of all significant covariates from the univariable analyses, both age and total radiation dose remained statistically significant in predicting POAF.
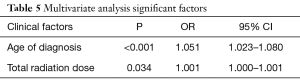
Full table
Discussion
Cardiac arrhythmias are a common sequela of esophagectomy and can be a host to postoperative morbidity and mortality. A recent study by Murthy and colleagues reported that POAF was significantly associated with infectious complications and anastomotic leakages (6). POAF also has implications for higher healthcare costs, as it is often associated with an increased hospital length of stay. Indeed, our patients with POAF did have a slightly longer median LOS than those without POAF (10.5 vs. 10.0, P=0.001) in agreement with published literature (15-17). Furthermore, postoperative arrhythmias can have longer lasting implications, as a study by Wells et al. suggested that POAF was associated with poorer long-term survival following esophagectomy (18).
While the exact pathogenesis of POAF has not been fully elucidated, these arrhythmias are believed to be the acute result of the inflammatory response associated with either surgery or preoperative chemoradiotherapy, as well as sympathetic activation, and/or damage to the vagus nerve (19). In our cohort of 677 patients receiving neoadjuvant therapy followed by esophagectomy, we observed a 14.9% incidence of POAF. This is comparable to other published studies which report a range of 8–22% (6-10).
Since POAF is associated with higher morbidity and mortality, predicting those at risk for developing this arrhythmia is of significant importance. Increasing age has been well documented as a risk factor for POAF (9,20,21), which we corroborate in our study. Other than advanced age, risk factors of cardiac arrythmias after esophagectomy varied among studies. Operative time has been posited to be correlated with increases in inflammatory markers such as IL-6 and C-reactive protein which may influence the pathogenesis of AF (22). We identified that patients with POAF had a longer operative time compared to those who did not (368 vs. 328 minutes, P=0.001), suggesting increased potential for inflammatory response and cardiac stress. Volume status has been under debate as a potential factor associated with arrhythmias (7,18,23,24). Specifically, we found that blood loss requiring blood transfusions was higher in patients with POAF that those without (19.2% vs. 10.6%, P=0.027) which has been observed in other studies (7,24). Unlike other groups however, we did not find that volume of fluid given over first 24 hours to be significantly different between the two patient populations (18). Further studies are required to better elucidate the influence of such factors on development of postoperative arrhythmia.
Recently, neoadjuvant therapy administration has been previously related to POAF (9,17). We did not find that neoadjuvant radiation type (2D vs. 3D vs. IMRT) was associated with POAF; however, we did observe that increasing radiation dose was associated with POAF. Radiation dose has not been well explored in its relationship with POAF, but available data suggests that radiation therapy induced heart complications are related to total cardiac dose, irradiated tissue volume and fraction size (25). Our group has previously reported that patients treated with dose escalated IMRT to 56 Gy had a statistically significant increase in POAF (26). In the case of EC, potential cardiac toxicity is concerning as cardiac dose is generally markedly higher given the location and/or higher total dose of the target area. Although a study by Colwell and colleagues reported that preoperative radiation was not a significant risk factor for POAF (16), this may be a result of the specific surgery (transhiatal with transcervical endoscopic esophageal mobilization) which impose different cardiac stresses when compared to a transthoracic esophagectomy. Current research in radiomics has also shown promise in predicting esophageal tumor response for chemoradiotherapy (27,28). Better understanding of the relationship between radiation dose and the degree of tumor response will allow for individualized radiation plans to potentially minimize the risk of POAF.
The high incidence of POAF after major oncologic thoracic surgery requires improved prevention and management strategies which can be accomplished with the development and collaboration of dedicated cardio-oncology programs (29-33). In the case of POAF, integrating a cardiologist into the multidisciplinary care team can provide optimal comprehensive patient care allowing for the safe but effective delivery of oncologic treatments.
This study has several limitations. They include its retrospective nature, potential patient selection/population bias, and any potential biases associated with different surgical techniques across operating surgeons. Although patient characteristics in the current study are consistent with those from previously published studies (9,17,18), the study sample is confined to a single institution. Additionally, subtle factors that may have influenced development of POAF such as electrolyte imbalances, hypoxia, and hypoglycemia were not available in our database to be analyzed. It is not possible to conclude that there are any causative relations between AF, increased radiation dose, and other clinical variables studied. The most reliable method of further defining such relations would be to perform a prospective study of AF in post-esophagectomy patients.
Conclusions
Increasing age and radiation dose were associated with the development of postoperative AF in this cohort. This study suggests that older patients or patients receiving higher neoadjuvant radiation dose should be monitored more closely in the postoperative setting, as these heightened risk factors should prompt early interventions. Integration of the cardiologist in multidisciplinary oncology care team can be beneficial in managing and monitoring these higher risk patients. Future study is required to determine if modification of current radiation techniques and cardiac dose constraints in this patient population may be warranted.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board at Moffitt Cancer Center (IRB number: MCC15030). As a retrospective chart review, written individual informed consent from subjects was waived by the IRB.
References
- Coleman HG, Xie SH, Lagergren J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018;154:390-405. [Crossref]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref]
- Gockel I, Exner C, Junginger T. Morbidity and mortality after esophagectomy for esophageal carcinoma: a risk analysis. World J Surg Oncol 2005;3:37. [Crossref]
- Ma JY, Wang Y, Zhao YF, et al. Atrial fibrillation after surgery for esophageal carcinoma: clinical and prognostic significance. World J Gastroenterol 2006;12:449-52. [Crossref]
- Murthy SC, Law S, Whooley BP, et al. Atrial fibrillation after esophagectomy is a marker for postoperative morbidity and mortality. J Thorac Cardiovasc Surg 2003;126:1162-7. [Crossref]
- Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg 2004;127:779-86. [Crossref]
- Hou JL, Gao K, Li M, et al. Increased N-terminal pro-brain natriuretic peptide level predicts atrial fibrillation after surgery for esophageal carcinoma. World J Gastroenterol 2008;14:2582-5.
- Mc Cormack O, Zaborowski A, King S, et al. New-onset atrial fibrillation post-surgery for esophageal and junctional cancer: incidence, management, and impact on short- and long-term outcomes. Ann Surg 2014;260:772-8; discussion 728. [Crossref]
- Stawicki SP, Prosciak MP, Gerlach AT, et al. Atrial fibrillation after esophagectomy: an indicator of postoperative morbidity. Gen Thorac Cardiovasc Surg 2011;59:399-405. [Crossref]
- Hannon N, Sheehan O, Kelly L, et al. Stroke associated with atrial fibrillation--incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis 2010;29:43-9. [Crossref]
- Jaakkola J, Mustonen P, Kiviniemi T, et al. Stroke as the First Manifestation of Atrial Fibrillation. PLoS One 2016;11:e0168010. [Crossref]
- Violi F, Soliman EZ, Pignatelli P, et al. Atrial Fibrillation and Myocardial Infarction: A Systematic Review and Appraisal of Pathophysiologic Mechanisms. J Am Heart Assoc 2016. [Crossref]
- Ruddox V, Sandven I, Munkhaugen J, et al. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol 2017;24:1555-66. [Crossref]
- Chen LT, Jiang CY. Impact of atrial arrhythmias after esophagectomy on recovery: A meta-analysis. Medicine (Baltimore) 2018;97:e10948. [Crossref]
- Colwell EM, Encarnacion CO, Rein LE, et al. Atrial fibrillation after transhiatal esophagectomy with transcervical endoscopic esophageal mobilization: one institution's experience. J Cardiothorac Surg 2018;13:73. [Crossref]
- Rao VP, Addae-Boateng E, Barua A, et al. Age and neo-adjuvant chemotherapy increase the risk of atrial fibrillation following oesophagectomy. Eur J Cardiothorac Surg 2012;42:438-43. [Crossref]
- Wells CI, Robertson JP, Campbell S, et al. Impact of atrial fibrillation on long-term survival following oesophagectomy: a 21-year observational study. ANZ J Surg 2018;88:E268-72. [Crossref]
- Maesen B, Nijs J, Maessen J, et al. Post-operative atrial fibrillation: a maze of mechanisms. Europace 2012;14:159-74. [Crossref]
- Day RW, Jaroszewski D, Chang YH, et al. Incidence and impact of postoperative atrial fibrillation after minimally invasive esophagectomy. Dis Esophagus 2016;29:583-8. [Crossref]
- Chin JH, Moon YJ, Jo JY, et al. Association between Postoperatively Developed Atrial Fibrillation and Long-Term Mortality after Esophagectomy in Esophageal Cancer Patients: An Observational Study. PLoS One 2016;11:e0154931. [Crossref]
- Takenaka K, Ogawa E, Wada H, et al. Systemic inflammatory response syndrome and surgical stress in thoracic surgery. J Crit Care 2006;21:48-53; discussion 53-5. [Crossref]
- Hahm TS, Lee JJ, Yang MK, et al. Risk factors for an intraoperative arrhythmia during esophagectomy. Yonsei Med J 2007;48:474-9. [Crossref]
- Onaitis M, D'Amico T, Zhao Y, et al. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg 2010;90:368-74. [Crossref]
- Giraud P, Cosset JM. Radiation toxicity to the heart: physiopathology and clinical data. Bull Cancer 2004;91 Suppl 3:147-53.
- Venkat PS, Shridhar R, Naghavi AO, et al. Dose escalated neoadjuvant chemoradiotherapy with dose-painting intensity-modulated radiation therapy and improved pathologic complete response in locally advanced esophageal cancer. Dis Esophagus 2017;30:1-9. [Crossref]
- Hou Z, Ren W, Li S, et al. Radiomic analysis in contrast-enhanced CT: predict treatment response to chemoradiotherapy in esophageal carcinoma. Oncotarget 2017;8:104444-54. [Crossref]
- Wu L, Wang C, Tan X, et al. Radiomics approach for preoperative identification of stages I-II and III-IV of esophageal cancer. Chin J Cancer Res 2018;30:396-405. [Crossref]
- Lenneman CG, Sawyer DB. Cardio-Oncology: An Update on Cardiotoxicity of Cancer-Related Treatment. Circ Res 2016;118:1008-20. [Crossref]
- Snipelisky D, Park JY, Lerman A, et al. How to Develop a Cardio-Oncology Clinic. Heart Fail Clin 2017;13:347-59. [Crossref]
- Johnson MN, Steingart R, Carver J. How to Develop a Cardio-oncology Fellowship. Heart Fail Clin 2017;13:361-6. [Crossref]
- Parent S, Pituskin E, Paterson DI. The Cardio-oncology Program: A Multidisciplinary Approach to the Care of Cancer Patients With Cardiovascular Disease. Can J Cardiol 2016;32:847-51. [Crossref]
- Fradley MG, Brown AC, Shields B, et al. Developing a Comprehensive Cardio-Oncology Program at a Cancer Institute: The Moffitt Cancer Center Experience. Oncol Rev 2017;11:340. [Crossref]