Effectiveness of radiation therapy in GIST: A case report
Introduction
Over the last decade, gastrointestinal stromal tumor (GIST) became the most commonly diagnosed mesenchymal tumor of the gastrointestinal tract (1,2). Population-based studies suggest an annual incidence of between 11 and 14.5 per million and a prevalence of 129 per million (3). The immunohistochemistry of GIST shows the presence of cell-surface antigen CD117 (KIT), which represents a defining characteristic of GIST (4-7). Immunostaining is essential to differentiate GISTs from other more rare mesenchymal tumors. Differential diagnosis includes leiomyosarcomas, leiomyomas and schwannomas (3). It is believed that GISTs arise from a neoplastic transformation of the intestinal pacemaker cells known as the interstitial cells of Cajal (ICC) (6,8).
Prior to 2002, the only available therapeutic option for patients with localized GISTs was surgical resection (9). Unfortunately, even when excised in negative surgical margins, the recurrence rate for lesions larger than 3 cm was found to be significant. Introduction of the first tyrosine kinase inhibitor, imatinib mesylate, has dramatically changed the management options available for GIST patients (10). The role of radiation therapy in the treatment of GISTs has not been documented (11). In the past, clinicians were reluctant to use radiation therapy due to concerns over the dose received by normal tissues, mostly the potential gastrointestinal toxicity. As such, radiation therapy has been utilized rarely, mostly for palliation purposes (12).
In this report, we describe the successful use of intensity modulated radiation therapy to treat an individual with large intra-abdominal GIST lesions (Figure 1), which were deemed unresectable. An initial attempt at systemic treatment with imatinib was not tolerated by the patient and did not produce a significant response.
Case presentation
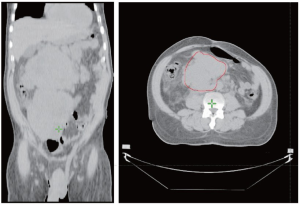
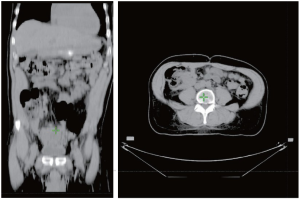
A 62 year-old African American male presented with complaints of lower abdominal pain for 3 months. He also had complaints of constipation, urinary frequency and weight loss for the same duration. Medical history was positive for hypertension and gallstones. His sister had an unknown malignancy. On physical examination, there was an ill-defined mass in the right lower abdomen. There was no lymphadenopathy or lower extremity edema. The rest of the physical examination was unremarkable. CT scan showed two huge, largely homogenous masses. The superior lesion measured 10.2 cm × 13.3 cm × 12.3 cm, located in the right upper quadrant, and the inferior mass was slightly larger, measuring 14.8 cm × 11.5 cm× 12.3 cm, and was located in the retroperitoneum (Figure. 1). Biopsy was performed. Histopathological examination revealed a gastrointestinal stromal tumor, epithelioid type, with high risk features (Figure 2). Patient was started on systemic therapy with imatinib mesylate (400 mg, po, qd) but developed fluid retention, protracted nausea and lower extremity edema on imatinib. Despite dose adjustments and drug holidays the imatinib was not tolerated, requiring discontinuation. Patient was referred for radiation therapy. Radiation therapy was administered conformally using initially a pair of left anterior oblique (LAO)/right posterior oblique (RPO) field arrangement to 43.2 Gy in 27 fractions, followed by a cone-down setup with an IMRT technique to a total of 63.4 Gy. Despite of the high dose, the radiation therapy was well tolerated and relieved the patient's symptoms with a dramatic reduction in tumor size demonstrated by CT scan (Figure 1,2).
Discussion
Gastrointestinal stromal tumors (GIST) account for less than 1% of all gastrointestinal (GI) tumors (13,14). In 1983, Mazur and Clark introduced the term GIST to describe a distinctive subgroup of GI mesenchymal tumor, which had neither neurogenic nor smooth muscle origin (15,16). It is believed that GISTs arise from a neoplastic transformation of the intestinal pacemaker cells known as the interstitial cells of Cajal (ICC) (8).
There is no strong predilection for either sex and these tumors can occur across a wide range of age groups (17). However, men are slightly more affected than women, and 75% of those diagnosed are over the age of 75 (18,19). So far, no link to environmental exposure, or relation with geographic location, ethnicity, or occupation has been established with incidence of GIST (20).
Morphologically, GISTs can appear as epithelioid, spindle cell, or a mixture of the two (21,22). The major histologic marker CD117, an epitope for the extracellular domain of KIT transmembrane receptor tyrosine kinase, stains positively in 95% of GISTs with a characteristic dot-like cytoplasmic pattern (23). Other important histological markers include CD34 (60-70%), ACAT (30-40%), DES (1-2%) and keratin (1-2%) (24).
GISTs show a diverse clinical presentation, with the most common symptoms being the presence of a mass or bleeding (1). The distribution of primary GISTs also varies throughout the gastrointestinal tract, with approximately 60-65% arising in the stomach, 20-25% in the small intestine, 5-10% in the colon or rectum and 5% in the esophagus (8,19).
The current treatment of choice for localized disease is surgical removal of the tumor with careful attention not to rupture the pseudocapsule. Unfortunately, less then 50% of patients have localized disease at diagnosis (18), and even when a curative resection is performed with clear margins the recurrence rate is approximately 50% (25). This recurrence rate can reach as high as 90% for large tumors with high mitotic rates.
In cases where the disease is extensive or the patient is not a surgical candidate, the choice of therapy is molecularly targeted chemotherapy with imatinib. Prior to the use of imatinib, chemotherapy results were dismal with reported success rates of 0-5% (18). The introduction of imatinib as a chemotherapeutic agent has greatly improved the treatment for non surgical candidates, with initial success rates of 70-90% (26). However, patients that do show an initial response are not cured and must stay on the drug indefinitely to prevent relapse (27). Furthermore, most patients eventually relapse and die of the disease (28,29).
Sunitinib malate, an oral agent inhibiting-multiple-tyrosine-kinases including KIT, PDGRFα as well as vascular endothelial growth factor receptor is recommended as second line of treatment for patients who experience disease progression while on imatinib treatment or who have life-threatening side effects. Although 20% of patients treated with Sunitinib have been stable for 2 or more years, age above 60 years, poor performance status, pretreatment with higher doses of imatinib and primary resistance to imatinib are predictors for poor response to treatment. Additionally, thrombocytopenia and hand-foot syndrome, frequently leads to poor tolerability (30).
The role of radiation therapy in the treatment of GISTs has not been documented and, in our opinion, it may be underutilized clinically. As stated previously, concerns over the potential side effects have led to a limited role of radiation therapy, mainly for palliative purposes, or in cases of intraperitoneal hemorrhage (1). It has been suggested that radiation may also sensitize GIST tumors to imatinib, although this has not been definitively established (31). The current radiation therapy techniques facilitate the administration of very high and effective doses to the target areas, while protecting efficiently surrounding vital structures. These new radiation technologies have not been explored in GIST tumors and deserve more study.
Conclusions
The enthusiasm for the targeted therapies in GIST tumors marginalized the use of the more conventional radiation therapy for GIST tumors. In our case, the judicious use of modern techniques of radiation produced an impressive response in a case of large intra-abdominal GIST masses, while being very well tolerated. It is too early to determine the length of response in this patient, yet similar techniques of radiation may prove even more efficient in earlier cases. We recommend, therefore, using radiation therapy more often not only for palliation purposes, but also for definitive treatment, with or without imatinib or sunitinib.
Footnote
No potential conflict of interest.
References
- Stamatakos M, Douzinas E, Stefanaki C, et al. Gastrointestinal stromal tumor. World J Surg Oncol 2009;7:61. [PubMed]
- Perez EA, Livingstone AS, Franceschi D, et al. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg 2006;202:623-629. [PubMed]
- Joensuu H, Kindblom LG. Gastrointestinal stromal tumors--a review. Acta Orthop Scand Suppl 2004;75:62-71. [PubMed]
- Badalamenti G, Rodolico V, Fulfaro F, et al. Gastrointestinal stromal tumors (GISTs): focus on histopathological diagnosis and biomolecular features. Ann Oncol 2007;18:vi136-vi140. [PubMed]
- Aubin F, Blanke CD. Metastatic gastrointestinal stromal tumors. Cancer Chemother Pharmacol 2011;67:S9-S14. [PubMed]
- Byrd D, Blancke C. Gastrointestinal stromal tumors. Liver metastases 2009;137-8.
- Gajiwala KS, Wu JC, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci USA 2009;106:1542-1547. [PubMed]
- Papaetis GS, Syrigos KN. Targeted therapy for gastrointestinal stromal tumors: current status and future perspectives. Cancer Metastasis Rev 2010;29:151-170. [PubMed]
- Eisenberg BL, Judson I. Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol 2004;11:465-475. [PubMed]
- Pisters PW, Patel SR. Gastrointestinal stromal tumors: current management. J Surg Oncol 2010;102:530-538. [PubMed]
- Pollock J, Morgan D, Denobile J, Williams J. Adjuvant radiotherapy for gastrointestinal stromal tumor of the rectum. Dig Dis Sci 2001;46:268-272. [PubMed]
- Siehl J, Thiel E. C-kit, GIST, and imatinib. Recent Results Cancer Res 2007;176:145-151. [PubMed]
- Howe JR, Karnell LH, Scott-Conner C. Small bowel sarcoma: analysis of survival from the National Cancer Data Base. Ann Surg Oncol 2001;8:496-508. [PubMed]
- van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer 2005;104:1781-1788. [PubMed]
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-519. [PubMed]
- Gupta P, Tewari M, Shukla HS. Gastrointestinal stromal tumor. Surg Oncol 2008;17:129-138. [PubMed]
- Arolfo S, Teggia PM, Nano M. Gastrointestinal stromal tumors: thirty years experience of an institution. World J Gastroenterol 2011;17:1836-1839. [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-58. [PubMed]
- Duffaud F, Salas S, Huyn T, Deville JL. Imatinib as the first and only treatment in Europe for adult patients at significant risk of relapse following gastrointestinal stromal tumor removal. Clinical and Experimental Gastroenterology 2010;3:41-47. [PubMed]
- Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol 2006;17:x280-x286. [PubMed]
- Papalambros A, Petrou A, Brennan N, Bramis K, Felekouras E, Papalambros E. GIST suture-line recurrence at a gastrojejunal anastomosis 8 years after gastrectomy: can GIST ever be described as truly benign? A case report. World J Surg Oncol 2010;8:90. [PubMed]
- Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477-489. [PubMed]
- Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol 1999;23:377-389. [PubMed]
- Urbańczyk K, Limon J, Korobowicz E, et al. Gastrointestinal stromal tumors. A multicenter experience. Pol J Pathol 2005;56:51-61. [PubMed]
- Pierie JP, Choudry U, Muzikansky A, et al. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg 2001;136:383-389. [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-480. [PubMed]
- Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective Multicentric Randomized Phase III Study of Imatinib in Patients With Advanced Gastrointestinal Stromal Tumors Comparing Interruption Versus Continuation of Treatment Beyond 1 Year: The French Sarcoma Group. J Clin Oncol 2007;25:1107-1113. [PubMed]
- Blay JY, Adenis A, Ray-Coquard I, Cassier PA, Le Cesne A. Is there a role for discontinuing imatinib in patients with advanced gastrointestinal stromal tumour? Curr Opin Oncol 2009;21:360-366. [PubMed]
- Nannini M, Biasco G, Pallotti MC, et al. Late recurrences of gastrointestinal stromal tumours (GISTs) after 5 years of follow-up. Med Oncol 2012;29:144-150. [PubMed]
- Nishida T, Omori T, Ueshima S. Evidence-based treatment of gastrointestinal stromal tumor (GIST) with tyrosine kinase inhibitors-imatinib and sunitinib. Gan To Kagaku Ryoho 2011;38:733-737. [PubMed]
- Ciresa M, D'Angelillo RM, Ramella S, et al. Molecularly targeted therapy and radiotherapy in the management of localized gastrointestinal stromal tumor (GIST) of the rectum: a case report. Tumori 2009;95:236-239. [PubMed]