Factors influencing response to neoadjuvant chemoradiation and outcomes in rectal cancer patients: tertiary Indian cancer hospital experience
Introduction
Colorectal cancer accounts for third most commonly diagnosed cancer in the world. In India, rectal cancer accounts for 6th most common digestive tract cancer as per cancer group projection from 2010 to 2020 (1).
Several randomized trials have demonstrated that neoadjuvant chemoradiotherapy (NACRT), as compared with postoperative CRT, downstaged tumours and improved local control, frequently permitting sphincter preservation in patients with low rectal tumours. Preoperative CRT was also associated with reduced toxicity (2-4). Though downstaging is achieved in majority of the tumours receiving long course NACRT the extent of downstaging and survival may vary from patient to patient. Many factors have been reported in literature that predicts response to (NACRT) of which advanced stage at presentation is the most important (5). Other factors such as mucinous and signet ring pathology also influence the outcomes and the data is scant. Therefore we studied the factors that can indicate the tumour response to preoperative CRT as well as the factors that influence disease free survival (DFS) and overall survival (OS) in our set of patients. In this study we present the results of a prospectively maintained data of rectal cancer patients who underwent NACRT and surgery at our centre.
Materials and methods
Patient selection
This study included 182 patients who underwent NACRT for biopsy proven rectal cancers at our institute between June 2006 and December 2010. The eligibility criteria included patient’s age more than 18 years, Karnofsky Performance Status (KPS) >70, resectable or unresectable rectal cancer who underwent chemoradiation as pre-operative treatment with different intent along with oral concurrent chemotherapy. The patients with distant metastasis at presentation, or who underwent short course of radiotherapy and those who did not receive concurrent chemotherapy or received altered fractionated RT were excluded from the study.
For initial staging evaluation all the patients underwent contrast enhanced computed tomography (CECT) abdomen and pelvis and chest X-ray. Additional MRI pelvis was done only in six patients. Multiplanar reformats were used to report T staging and involvement of mesorectal fascia (MRF) which was reported in most of the cases. The tumors were labelled as T3 if there was perirectal fat stranding and T4 if there was definite invasion of the surrounding organ. A thorough clinical examination including a careful per-rectal examination both by an onco-surgeon as well as radiation oncologist was performed. Distance from anal margin was assessed by digital rectal examination in majority of the patients. In addition colonoscopic evaluation and biopsy, serum carcino-embryonic antigen (CEA) estimation was also done.
Treatment protocol
Radiotherapy was given to a dose of 45-50.4 Gy in conventional fractionation (180-200 cGy per fraction, one fraction per day and five fractions per week) with treatment ranging between 5-5.5 weeks. All the patients received oral capecitabine concurrently to a dose of 850 mg/m2 in twice daily. Post chemoradiation at 6 weeks, patients were assessed by per-rectal examination and pelvic imaging and surgery was planned if deemed resectable. All the eligible patients underwent complete total mesorectal excision with either low anterior resection or abdominoperineal resection (APR) with permanent colostomy. The post-operative specimen was analysed in detail for tumour size, nodal stage, pathological response, margin status including circumferential resection margin, tumour regression grade (TRG) score using Mendard’s scoring were assessed. Every attempt was made by the pathologist to retrieve maximum nodes possible. All patients were planned for adjuvant chemotherapy. If all the nodes were negative in the resected specimen they were planned for 6 cycles of adjuvant chemotherapy of capecitabine alone, and for node positive disease 6 cycles of CAPOX (capecitabine 1,000 mg/m2 and injection oxaliplatin) was advised. Patient who had unresectable tumour post CRT were given further chemotherapy (preferably CAPOX) and were continued to assess for operability every 3 months or till disease progression and were taken up for surgery if deemed operable.
Follow up
All patients were reviewed weekly during CRT for treatment compliance, toxicities and need for symptomatic management. The toxicities were recorded as per common terminology criteria for adverse events (CTCAE) version 3 criteria. Weekly blood counts were done to monitor haematological parameters. Post-surgery the further follow up were scheduled 3 monthly for the first 2 years and then 6 monthly for 5 years. Complete blood count liver function tests and renal function tests with CEA were done at each follow up. Colono videoscope examinations were performed at 1-year postoperatively and then once every 3 years. Recurrence was diagnosed pathologically by surgical resection, biopsy or cytology and/or radiological findings showing an increase in tumour size over time.
Statistical analysis
DFS was calculated from the date of registration to the date of disease recurrence and OS till the date of last follow up. DFS and OS were calculated using Kaplan-Meir actuarial method. The analysis used standard log rank test with an intention to treat basis. Univariate and multivariate analysis were performed to find out factors affecting both DFS and OS. The patients who underwent surgical resection after chemo-radiation the factors influencing DFS and OS in both univariate and multivariate analysis were observed. All the statistical analysis was performed using SPSS version 18.
Results
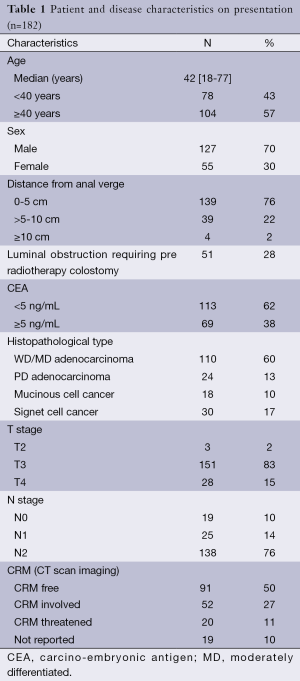
Total 182 patients underwent NACRT. The patient and disease pre-treatment characteristics are being summarized in Table 1. The median age was 42 years and there were more males than females (70% vs. 30%) and 38% of the patients had MRF threatened or involved at presentation.
Full table
Following the institutional protocol 178 patients completed NACRT, of these 131 (72%) of the patients underwent surgical resection. Twenty nine patients continued to have unresectable disease post chemo radiation were given palliative chemotherapy. Nine (5%) patients refused surgery due to fear of colostomy and 11 (6%) patients were found to have distant metastasis on imaging at 6 weeks so were given palliative chemotherapy. Two patients died of myocardial infarction post CRT. Capecitabine based adjuvant chemotherapy was received by 112 (85%) of the patients.
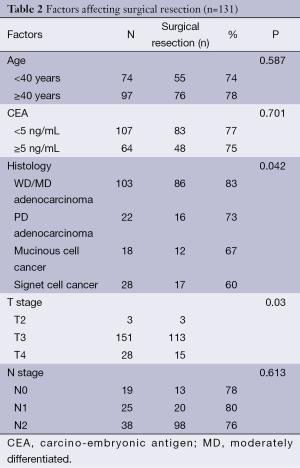
The major factors affecting tumour downsizing and subsequent R0 surgical resection were advanced T stage and signet ring cell pathology (Table 2).
Full table
Acute toxicity
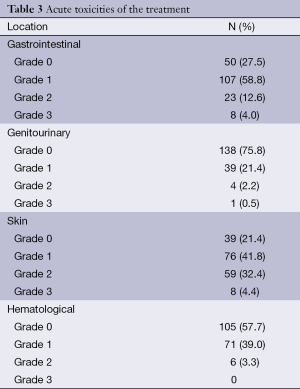
During the chemo-radiation protocol most of the patients tolerated treatment well. The main acute side effects are being summarized in Table 3. The toxicity grading was done as per the CTCAE version 3. In gastrointestinal toxicity majority had grade 1 anorexia or diarrhoea. The skin toxicity was limited to perianal skin and groins. In haematological toxicity a large number had grade 1 anaemia which improved with regular haematinics.
Full table
The surgical complications were few and mostly due to wound infection in 10 patients, anastomotic leak in 2 patients and urinary leak from perineal wound in 1 patient respectively. The wound healing rate was mostly within time and only 10 (17.2%) patients had delayed wound healing.
Survival
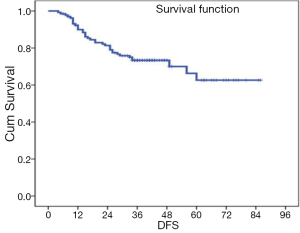
The median follow up for all the patients was 43 [4-88] and 47 [8-88] months for the patients who underwent resection. Two patients died of acute myocardial infarction post CRT. The analysis of tumour control and survival has been restricted to the 131 patients who underwent R0 or R1 resection. Of the 131 patients, 91 (72%) are alive and disease free. At 5 years the DFS and OS was 60% and 77% (Figures 1,2). For the patients who did not undergo surgery their median survival was 13 [3-78] months since the disease progressed locally and distally and most of them died by 2 years.
Factors affecting local and distant failures
Of the 131 operated patients 9 (7%) failed locally and 4 (3%) failed locally and distally, while the majority 26 (20%) failed distally. The majority of distant failures were in lung (30%), liver (23%) and paraaortic nodes (20%). The details of patterns of failure and the factors influencing is being represented in (Table 4). Of the 16 patients whose circumferential resection margin was positive, 10 (62%) failed. Four locally and 3 distant metastasis and 2 failed both local and distant.
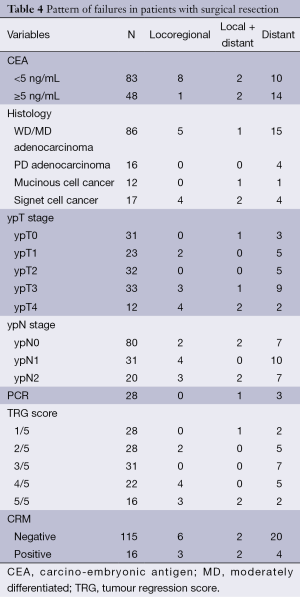
Full table
Of the 28 (20.3%) patients achieving complete pathological (pCR) response, only 1 failed locally while 3 patients had distant metastasis.
Factors affecting DFS and OS
Using Kaplan-Meier actuarial method both univariate and multivariate analyses were performed in terms of DFS and OS (Tables 4,5).
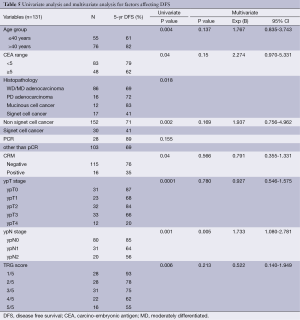
Full table
In univariate analysis DFS was inferior in pre RT CEA levels of ≥5 ng/mL, signet ring cell pathology, R1 resection, and lack of tumour downstaging represented by pathological T & N stage and higher TRG scores. On multivariate analysis the factors independently affecting inferior DFS were only the pathological node positivity.
OS was inferior in patients with young age of patients, pre RT CEA levels of ≥5 ng/mL, higher pathological T, N stage and TRG scores. In multivariate analysis the factors independently affecting OS were only signet ring cell pathology and a trend towards worse survival for the patients presenting with pre RT CEA levels of ≥5 ng/mL.
Multivariate analysis for DFS and OS being represented in Tables 5,6.
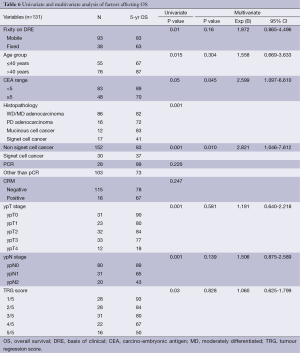
Full table
OS, overall survival; DRE, basis of clinical; CEA, carcino-embryonic antigen; MD, moderately differentiated; TRG, tumour regression score.
Discussion
The response to NACRT can vary in locally advanced rectal cancers and thus affect survival. Therefore it is very important to predict these factors before starting NACRT so as to deliver the appropriate treatments. In the present study the factors predicting poor OS were locally advanced tumours with signet ring cell pathology and baseline high CEA levels.
The most common histopathological type of rectal cancer being adenocarcinoma and in accordance to world data our study also had 2/3rd patients with the same. Rectal cancers with signet ring cell morphology is reported as rare in most of the literature, the incidence ranging from 1-13% in most of the studies (6-8). In our study the signet ring cell carcinomas were seen in 17% patients and majority (19 patients) were in younger age group of <40 years. The three main concerns regarding signet ring cell carcinomas being younger age at presentation, locally advanced and unresectable to begin with and hence poor survival. Chang et al. in Stanford University studied these early onset sporadic rectal cancers and found that signet ring cell differentiation was the major factor leading to poor outcomes in these patients (8).
Patients having signet cell pathology only 17 (56%) underwent downstaging and subsequent surgical resection. Among the operated patients again 11 (65%) of them failed both locally and distally leading to a significantly poorer OS when compared to moderately differentiated (MD) adenocarcinoma. In multivariate analysis signet ring cell carcinoma stood out to be an independent poor prognostic factor for an inferior OS but not for DFS. This indicates that after recurrence the salvage therapy is more effective for prolonging survival in patients with non-signet ring cell tumours compared to the signet cell ones, therefore there is a need for more aggressive salvage strategies for signet ring cell cancers.
A report from National Cancer Database on 244,794 colorectal cases from America reported that signet ring cell histology was independently associated with higher risk of death (HR 1.42, 95% CI, 1.33-1.51) (9). The Korean National registry also reported their SEER database of signet ring cell carcinoma and found to have higher grade and worse DFS (10). In accordance to our series similar data were available regarding higher disease burden and primarily unresectable stage of rectal signet cell carcinoma (11). This was similar to a German series of 34 patients with 65% being primarily unresectable (12).
Another, rather contradictory study among Indian population stated signet ring cell carcinomas to be associated with better pCR rates and better survival (13). This prompts us to study prospectively with a larger population about actual behaviour of signet ring cell carcinomas and whether their histology per se or their late presentation is actually responsible for the worse outcome.
Several studies have proved the importance of serum CEA level as tumour marker for rectal cancers and its significant impact upon resectability, DFS and OS suggesting it could predict occult distant metastasis as well as predict CRT response and serves as an important marker in patient outcomes (14,15). It inhibits cell death by causing a loss of anchorage to the extracellular matrix. Tumour cells containing a high density of CEA are resistant to radiation (16). In the present study, pre-treatment CEA level of ≥5 ng/mL was associated with worse DFS and OS and was an independent factor predicting OS on multivariate analysis. Similarly in a study from Korea, the 5-year DFS rate was significantly higher in the CEA <5 ng/mL group than in the CEA ≥5 ng/mL group (73.2% vs. 60.9%, P=0.002) (17).
Pathological T and N stage are known to be important factors influencing DFS and OS (18). Pathological nodal staging was an independent prognostic marker in most of the studies analyzing the postoperative predictive factors for survival (4,19). In the present study patients with positive nodes in the pathological specimen had significantly higher rates of distant failures leading to poorer DFS which was also a significant factor in multivariate analysis.
Tumour regression is categorized on the basis of a semi quantitative assessment comparing the amount of viable tumour with the amount of fibrosis TRG and is a good indicator of tumour response (20). This was reflected in our patients where the lower TRG score was associated with better survival in the univariate analysis but was not a significant factor on the multivariate analysis.
Due to resource constraint most of the patients were staged on the basis of clinical (DRE) and CT scan imaging. Though preoperative staging with MRI is considered the standard of care there are studies in the literature which suggest that CECT can be used for initial staging in the resource constrained environment (21,22). Rectal cancer mostly present in younger age group in India, a factor which is neither hereditary nor diet related (1) (Table 1). In our study about 43% of the patients were below 40 years and the negative effect of age upon disease free and OS has recently been highlighted in few studies where the younger patients had statistically significant difference in survival and fared poorly (7,23). Similarly in our study patients with age less than 40 years had 5 years DFS and OS (61% and 68% vs. 68% and 88%) respectively and compared to the other group results were statistically significant (P=0.001 and 0.009). But in multivariate analysis this was not significant. This was in contrast to another study were advanced age was associated with worse OS (24).
Overall the survival outcomes of our patients are comparable with the data in the literature. Besides this the study highlights the fact that locally advanced tumors having CRM threatened margins with high CEA levels and signet ring cell morphology are independent good predictive markers for poorer survival pre NACTRT. Therefore more aggressive neoadjuvant treatment strategies both in terms of locoregional and systemic should be employed to treat these aggressive tumours.
Patients with positive nodes in the surgical specimens have a high chance of failures more so distally. Though most of the patients in this study received adjuvant chemotherapy still almost half of the node positive patients failed distally. Therefore pN stage serves as a good marker post neoadjuvant treatment, indicating a need for more aggressive adjuvant chemotherapy regimens to tackle distant metastasis (25,26).
Conclusions
The outcome of our patients were similar to World Literature and signet ring cell morphology, initial stage, pre-treatment CEA level, and pathological nodal staging all were influential in determining survival. Besides this the study also highlights the fact that locally advanced tumours with signet cell histology are aggressive and fare poorly in terms of achieving R0 resection and poorer survival compared to the other histologies, therefore more intensive chemo radiation strategies like neoadjuvant chemotherapy followed by chemo radiation and aggressive surgical resections can be attempted. The possibility of radiotherapy dose escalation and addition of newer biological agents in conjunction would be the future direction.
Acknowledgements
We would like to thank all the authors for their significant contribution towards the manuscript. We would also thank the authors whose articles were selected for reference and was a constant help while preparing the manuscript.
Disclosure: The authors declared no conflict of interest.
References
- Mohandas KM, Jagannath P. Epidemiology of digestive tract cancers in India. VI. Projected burden in the new millennium and the need for primary prevention. Indian J Gastroenterol 2000;19:74-8. [PubMed]
- Silberfein EJ, Kattepogu KM, Hu CY, et al. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol 2010;17:2863-9. [PubMed]
- Dhadda AS, Zaitoun AM, Bessell EM. Regression of rectal cancer with radiotherapy with or without concurrent capecitabine--optimising the timing of surgical resection. Clin Oncol (R Coll Radiol) 2009;21:23-31. [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [PubMed]
- Yoon SM, Kim DY, Kim TH, et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2007;69:1167-72. [PubMed]
- Börger ME, Gosens MJ, Jeuken JW, et al. Signet ring cell differentiation in mucinous colorectal carcinoma. J Pathol 2007;212:278-86. [PubMed]
- Chen JS, Hsieh PS, Chiang JM, et al. Clinical outcome of signet ring cell carcinoma and mucinous adenocarcinoma of the colon. Chang Gung Med J 2010;33:51-7. [PubMed]
- Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012;25:1128-39. [PubMed]
- Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012;19:2814-21. [PubMed]
- Kang H, O'Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48:1161-8. [PubMed]
- Anthony T, George R, Rodriguez-Bigas M, et al. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol 1996;3:344-8. [PubMed]
- Bittorf B, Merkel S, Matzel KE, et al. Primary signet-ring cell carcinoma of the colorectum. Langenbecks Arch Surg 2004;389:178-83. [PubMed]
- Jayanand SB, Seshadri RA, Tapkire R. Signet ring cell histology and non-circumferential tumors predict pathological complete response following neoadjuvant chemoradiation in rectal cancers. Int J Colorectal Dis 2011;26:23-7. [PubMed]
- Moreno García V, Cejas P, Blanco Codesido M, et al. Prognostic value of carcinoembryonic antigen level in rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2009;24:741-8. [PubMed]
- Moureau-Zabotto L, Farnault B, de Chaisemartin C, et al. Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2011;80:483-91. [PubMed]
- Ordoñez C, Screaton RA, Ilantzis C, et al. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 2000;60:3419-24. [PubMed]
- Lee JH, Kim SH, Jang HS, et al. Preoperative elevation of carcinoembryonic antigen predicts poor tumor response and frequent distant recurrence for patients with rectal cancer who receive preoperative chemoradiotherapy and total mesorectal excision: a multi-institutional analysis in an Asian population. Int J Colorectal Dis 2013;28:511-7. [PubMed]
- Pacelli F, Sanchez AM, Covino M, et al. Improved outcomes for rectal cancer in the era of preoperative chemoradiation and tailored mesorectal excision: a series of 338 consecutive cases. Am Surg 2013;79:151-61. [PubMed]
- Huebner M, Wolff BG, Smyrk TC, et al. Partial pathologic response and nodal status as most significant prognostic factors for advanced rectal cancer treated with preoperative chemoradiotherapy. World J Surg 2012;36:675-83. [PubMed]
- Bujko K, Kolodziejczyk M, Nasierowska-Guttmejer A, et al. Tumour regression grading in patients with residual rectal cancer after preoperative chemoradiation. Radiother Oncol 2010;95:298-302. [PubMed]
- Maizlin ZV, Brown JA, So G, et al. Can CT replace MRI in preoperative assessment of the circumferential resection margin in rectal cancer? Dis Colon Rectum 2010;53:308-14. [PubMed]
- Ahmetoğlu A, Cansu A, Baki D, et al. MDCT with multiplanar reconstruction in the preoperative local staging of rectal tumor. Abdom Imaging 2011;36:31-7. [PubMed]
- Engineer R, Mohandas KM, Shukla PJ, et al. Escalated radiation dose alone vs. concurrent chemoradiation for locally advanced and unresectable rectal cancers: results from phase II randomized study. Int J Colorectal Dis 2013;28:959-66. [PubMed]
- Bansal V, Bhutani R, Doval D, et al. Neo adjuvant chemo-radiotherapy and rectal cancer: can India follow the West? J Cancer Res Ther 2012;8:209-14. [PubMed]
- Mathis KL, Nelson H, Pemberton JH. Can unresectable colorectal cancer be cured? Adv Surg 2009;43:211-9. [PubMed]
- Mathis KL, Nelson H, Pemberton JH, et al. Unresectable colorectal cancer can be cured with multimodality therapy. Ann Surg 2008;248:592-8. [PubMed]