Low bone mineral density linked to colorectal adenomas: a cross-sectional study of a racially diverse population
Introduction
Colorectal adenocarcinoma is the third most common cancer and the third leading cause of cancer-related deaths in both men and women in the United States (1). In fact, the American Cancer Society estimates 50,310 deaths due to colorectal cancer and 96,830 new cases of colon cancer will occur in the United States in 2014 (1). Worldwide, colorectal carcinoma was the second most prevalent cancer among males and third among females in 2012 (2). Most colon cancers arise when normal colonic mucosa undergoes genetic mutations, leading to hyper-proliferation and the development of pre-malignant adenomas, which subsequently undergo further mutations in genes such as p53 to become carcinomas (3,4). The death rate from colorectal adenocarcinoma has been dropping in both men and women with a reported 30% decrease in the U.S. for ages 50 years and older largely due to improved treatment and screening allowing colorectal cancers to be detected at earlier, more curable stages (5). Designing better protocols for targeted screening for colorectal cancer involves the identification of risk factors associated with colorectal cancer. Previously identified risk factors include increasing age, smoking, alcohol, obesity, and diabetes (1,3,6-9). Additionally, African Americans are more likely to be diagnosed with colorectal cancers at advanced (non-curable) stages, have the highest incidence of colorectal cancer of any racial or ethnic group, and have a younger mean age at diagnosis and lower survival compared to whites (10). Further epidemiological studies are needed to understand the true cause and measures for prevention of this outcome, a feat which is particularly important considering the expected population growth of certain non-white racial groups.
Like colorectal cancer, osteoporosis is a widely prevalent disease worldwide that correlates with aging populations. Few studies have investigated correlations between these two diseases, or the effect of bone mineral density (BMD) on the likelihood of developing colorectal adenomas. BMD reflects an individual’s lifetime exposure to several factors, including calcium and vitamin D. It has already well known that higher dietary intake of calcium and circulating levels of Vitamin D metabolites have been inversely associated with colorectal carcinoma and adenoma risk (11-14). BMD and colorectal cancer continue to co-exist as major co-morbidities in patient populations around the world (15,16). Few clinical studies performed to date clearly demonstrate that low BMD, osteopenia, or osteoporosis in populations coincide with greater likelihood of colorectal adenomas (17,18). Given these findings, we set out to investigate the association between osteopenia and/or osteoporosis and colorectal adenomas in patients from a diverse New York community hospital.
Methods
Following Institutional Review Board (IRB) approval at Nassau University Medical Center, a 530-bed tertiary care teaching hospital in East Meadow, New York, a cross-sectional observational study was performed between November 2009 and March 2011. We established a database of 200 patients who underwent screening colonoscopies and bone density scan (dual X-ray absorptiometry). A total of 83 patients with osteoporosis (defined as having a T score of –2.5 or below) and 67 patients with osteopenia (defined as a T score less than -1.0 but greater than –2.5) were examined by colonoscopy for the presence of colorectal adenomas. Data were collected on patient demographics and potential risk factors for adenoma including age, sex, race, body mass index (BMI), diabetes, hypertension, dyslipidemia, tobacco use, alcohol use, and family history of colorectal cancer. Recorded colonoscopy findings included presence of adenoma, adenoma ≥10 mm, adenoma ≥2 mm, adenoma present in the proximal colon, adenoma present in the distal colon, largest adenoma in the proximal colon, and largest adenoma in the distal colon.
Statistical analysis
Comparisons between categorical variables were made using a chi square test, and ANOVA was used for comparisons between continuous variables. Unconditional logistic regression was used to generate odds ratios adjusted for age, sex, race, BMI, alcohol, tobacco, and history of diabetes, hypertension, or hyperlipidemia and their 95% confidence intervals (CI) comparing bone mineral densities with presence of colorectal adenomas. Statistical analyses were performed with SAS 9.3 software.
Results
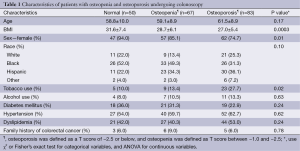
Table 1 shows the demographic characteristics of the patients with normal BMD, osteopenia, and osteoporosis undergoing either screening or diagnostic colonoscopy. The mean age among those with osteoporosis was higher than for those with osteopenia and normal bone mineral density (61.5 vs. 59.1 vs. 58.8, P<0.17, respectively). A larger portion of osteoporotic patients (74.7%, n=62) vs. osteopenic patients (85.1%, n=57), or patients with normal bone mineral density (94.0%, n=47) were female. Patients with osteoporosis, osteopenia, and normal bone mineral density differed in metabolic and environmental factors as well, including BMI (P=0.0003) and smoking status (P=0.02).They were not observed to differ in the presence of hypertension (P=0.62), dyslipidemia (P=0.24), diabetes mellitus (P=0.24), alcohol use (P=0.63), or family history of colorectal cancer (P=0.78).
Full table
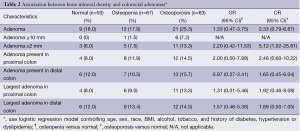
Table 2 shows the association between BMD and colonoscopy findings, adjusted for age, sex, race, BMI, alcohol or tobacco use, history of diabetes, and hypertension. A larger proportion of osteoporotic patients (25.3%) had an adenoma found on colonoscopy compared to the osteopenic patients (17.9%) and patients with normal bone mineral density (18.0%), although this difference did not reach statistical significance. However, looking at patients with synchronous adenomas, a larger proportion of osteoporotic patients (13.3%) had two or more adenomas on colonoscopy than did osteopenic patients (7.5%) or patients with normal bone mineral density (6.0%; OR, 5.12; 95% CI: 1.02-25.81). Although not reaching statistical significance, osteoporotic patients were more likely than patients with either osteopenia or normal bone mineral density to have an adenoma present in the proximal colon (14.5% vs. 11.9% vs. 8%, respectively) and largest adenoma present in the proximal colon (13.3% vs. 9.0% vs. 8.0%, respectively). Similarly, osteoporotic patients were also more likely to have an adenoma in the distal colon compared to osteopenic and normal BMD patients (15.7% vs. 10.5% vs. 12%), as well as largest adenoma present in the distal colon (14.5% vs. 13.4% vs. 12.0%, respectively).
Full table
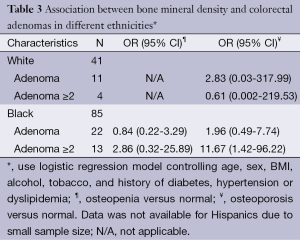
Table 3 shows the association between bone mineral density and colorectal adenomas in different ethnicities, adjusted for age, sex, BMI, alcohol or tobacco use, history of diabetes, and hypertension. A statistically significant portion of osteoporotic African Americans were more likely than normal controls to have two or greater adenomas (OR, 11.67; 95% CI: 1.42-96.22). No such relationship was found to be true for whites.
Full table
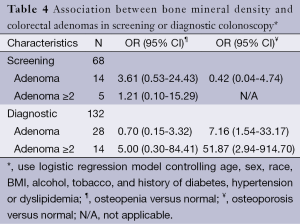
Table 4 shows the association between BMD and colorectal adenomas in screening or diagnostic colonoscopy, adjusted for age, sex, race, BMI, alcohol or tobacco use, history of diabetes, and hypertension. A statistically significant portion of osteoporotic individuals were more likely than normal controls to have adenomas on diagnostic colonoscopy, either one (OR, 7.16; 95% CI: 1.54-33.17) or two or more adenomas (OR, 51.87; 95% CI: 2.94-914.70). In fact, on diagnostic colonoscopy, osteoporotic patients were more than seven times more likely to have two or more adenomas compared to just a single adenoma (OR, 51.87 vs. 7.16). No such relationship was found on screening colonoscopy.
Full table
Discussion
To our knowledge, this is one of the first studies assessing the relationship between low bone mineral densities and colorectal adenoma prevalence in a racially diverse U.S. population. We found that osteopenia and osteoporosis were associated with a statistically significant increased incidence of synchronous adenomas, and that adenoma detection rates, presence of adenomas in the proximal and distal colon, as well as largest adenoma in the distal and proximal colon were higher in the osteoporotic cohort although not reaching statistical significance.
Two previous retrospective studies have been performed looking at the association of bone mineral density and colorectal adenomas (17,18). One performed at the University Hospitals in Cleveland, Ohio gathered data from 167 patients who underwent colonoscopy. Patients in the highest percentile of total body BMD (>1.294 g/cm2) and in the middle percentile (≥1.167 to ≤1.294 g/cm2) compared to those with a total body BMD in the lowest percentile (<1.167 g/cm2) had a lower risk of colorectal adenomas [highest vs. lowest percentile: OR, 0.29 (95% CI: 0.10-0.84); middle vs. lowest percentile: OR, 0.26 (95% CI: 0.08–0.80); P-trend=0.02]. Stratification by gender revealed that this association was more pronounced in women (highest (>1.280 g/cm2) vs. lowest (<1.130 g/cm2) percentile: OR, 0.08 (95% CI: 0.01-0.70); middle (≥1.130 to ≤1.280 g/cm2) vs. lowest percentile: OR, 0.15 (95% CI: 0.04-0.94); P-trend=0.02) even after excluding hormone replacement therapy users (highest (>1.295 g/cm2) and middle (≥1.132 to ≤1.295 g/cm2) vs. lowest (<1.132 g/cm2) percentile: OR, 0.17 (95% CI: 0.03-0.97); P-trend=0.04). The authors of the study concluded that BMD is inversely associated with colorectal adenomas, especially in women (17). Limitations of the Cleveland study include that it was a retrospective study, precluding a definitive understanding of the temporal relationship of BMDs and colorectal carcinomas, which would have perhaps allowed a hypothesis about causation. Furthermore, the study sample was small. Strengths of the study included minimal selection bias, as those who participated in the study have similar demographic characteristics to those who did not.
Another retrospective study was done at Gangdong Kyung Hee University Hospital in Korea (18). This was a cross-sectional study involving 992 patients who presented for a routine health check-up and underwent DEXA scanning for BMD and initial screening colonoscopy on the same day. A multivariate analysis on the prevalence of colorectal adenomas according to BMD level concluded that osteoporosis was associated with an increased risk of colorectal adenomas in women older than 50 years. Limitations of the study included possible selection bias, as it included only women over 50 years old, but the authors state that this bias was likely minimal, as adenoma detection rates found in the study were similar to community-wide prevalence in Korea. Furthermore, it was a retrospective study, which the authors acknowledge prevented full and accurate collection of variables. Strengths of the study include a large study sample, good data collection with questionnaires conducted by trained nurses, and data collection from apparently health women, allowing accurate data representation of middle-age and elderly women in the general public.
Strengths of our study include a racially diverse patient population, which mirrors current diversity and projected trends (3). Furthermore, our study did control for multiple confounding data, despite being retrospective. Limitations include a small sample size and wide odds ratio CIs, most likely a result of our small sample size. Due to the small sample size, data was not available for Hispanics, who nonetheless comprise a growing part of the U.S. population (3). Our study, like the others performed prior to it, is retrospective, preventing control of all potentially confounding factors, including risk factors for colorectal cancer not accounted for in our study. The retrospective nature of our study also prevents us from being able to accurately understand the temporal relationship of BMDs and the presence of adenomas. Larger, prospective studies are warranted to adjust for the limitations of our study.
Calcium and vitamin D modify colorectal cancer risk through altering levels of cellular differentiation and apoptosis in colonic epithelium. It is hypothesized that higher calcium levels exert an anti-neoplastic effect in the colonic epithelium through induction of apoptosis (19,20). Vitamin D exerts a direct effect through regulation of growth factors and cytokine synthesis and signaling as well as modulation of the cell cycle, apoptosis, and differentiation (21). It has been previously demonstrated to have antiproliferative properties against many cancer cell lines, such as breast, prostate and even colon cancer (22-28). A previous study revealed that a vitamin D analogue, 1,25-dihydroxyvitamin D3, has antiproliferative effects in colorectal adenocarcinoma cells via the vitamin D receptor, while another demonstrated that vitamin D and its metabolites inhibit proliferation in normal and premalignant rectal epithelium, suppressing growth in colorectal cancer cell lines (24,25). These findings highlight the ability of these two dietary supplements to play a role in the prevention of carcinogenesis. BMD reflects an individual’s lifetime exposure to calcium and vitamin D, with a deficiency in these two supplements playing a part in low BMD and possibly increased risk of cancer.
BMD may be particularly appealing as a biomarker for colorectal cancer risk given that the levels it collectively reflects are not reliant on patient reporting and are therefore not subject to measurement error and bias. Furthermore, even if objective individual levels of calcium, vitamin D, and estrogen are obtained through serum measurement, they only reflect levels at a “snap shot” of time, and “snap-shot” levels of these substances are unlikely to influence colorectal cancer risk in the same way that cumulative levels of these substances in the body over a longer period of time would. BMD is a biomarker for these substances over a longer period of time and is therefore a better measurement than serum when assessing colorectal cancer risk. BMD may be particularly useful as a biomarker in women, who are more likely to undergo DEXA scanning and have been reported to have decreased rates of adenoma detection compared to men (17). Overall, BMD is non-invasive and efficient, and may help identify patients who may benefit from earlier colorectal cancer screening.
A disproportionate number of osteoporotic and osteopenic African Americans have multiple adenomas on diagnostic colonoscopy, perhaps reflecting a need for better calcium or vitamin D intake and physical activity among members of this population, more widespread and/or earlier screening of this population for bone mineral density, as well as improved public health and government efforts to inform this population of increased risk. While less access to care may play a large role in the increased prevalence of low bone mineral density and colorectal carcinoma mortality among African Americans, especially those belonging to lower socioeconomic groups, further investigations need to be done to understand all the factors responsible for these phenomena. The identification of such factors would have important implications regarding screening for at-risk populations.
In summary, our study demonstrates a clear association between lower bone mineral density and the presence of colorectal adenomas that can subsequently develop into carcinomas. Our findings, confirmed by further studies, may influence colonoscopy screening guidelines for those with lower BMD, with particular relevance to women, given the larger prevalence of decreased bone mineral density in women compared to men. Further studies with larger population base would need to be done to confirm our findings as well as to further investigate racial profiling of colorectal adenoma prevalence, guiding any necessary interventions to help the subpopulations most at risk for disease.
Acknowledgements
All the authors contributed equally to the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- American Cancer Society. What are the key statistics about colorectal cancer? Available Online: www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-key-statistics
- Marques AM, Turner A, de Mello RA. Personalizing medicine for metastatic colorectal cancer: current developments. World J Gastroenterol 2014;20:10425-31. [PubMed]
- Davis-Yadley AH, Lipka S, Shen H, et al. Ethnic disparities in the risk of colorectal adenomas associated with aspirin and statin use: a retrospective multiethnic study. J Gastrointest Oncol 2014;5:112-8. [PubMed]
- Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer 1992;70:1727-31. [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [PubMed]
- Crockett SD, Long MD, Dellon ES, et al. Inverse relationship between moderate alcohol intake and rectal cancer: analysis of the North Carolina Colon Cancer Study. Dis Colon Rectum 2011;54:887-94. [PubMed]
- Pedersen A, Johansen C, Grønbaek M. Relations between amount and type of alcohol and colon and rectal cancer in a Danish population based cohort study. Gut 2003;52:861-7. [PubMed]
- Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679-87. [PubMed]
- Chao A, Thun MJ, Jacobs EJ, et al. Cigarette smoking and colorectal cancer mortality in the cancer prevention study II. J Natl Cancer Inst 2000;92:1888-96. [PubMed]
- Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol 2005;100:515-23; discussion 514. [PubMed]
- Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 2004;96:1015-22. [PubMed]
- Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer 2009;61:47-69. [PubMed]
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 2009;30:113-25. [PubMed]
- Baron JA, Beach M, Mandel JS, et al. Calcium supplements and colorectal adenomas. Polyp Prevention Study Group. Ann N Y Acad Sci 1999;889:138-145. [PubMed]
- Marques AM, Turner A, de Mello RA. Personalizing medicine for metastatic colorectal cancer: current developments. World J Gastroenterol 2014;20:10425-31. [PubMed]
- Cooper C. Osteoporosis research thrives: IOF WCO-ECCEO10. Expert Rev Pharmacoecon Outcomes Res 2010;10:367-70. [PubMed]
- Nock NL, Patrick-Melin A, Cook M, et al. Higher bone mineral density is associated with a decreased risk of colorectal adenomas. Int J Cancer 2011;129:956-64. [PubMed]
- Lim JU, Cha JM, Lee JI, et al. Osteoporosis is associated with the risk of colorectal adenoma in women. Dis Colon Rectum 2013;56:169-74. [PubMed]
- Penman ID, Liang QL, Bode J, et al. Dietary calcium supplementation increases apoptosis in the distal murine colonic epithelium. J Clin Pathol 2000;53:302-7. [PubMed]
- Fedirko V, Bostick RM, Flanders WD, et al. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res (Phila) 2009;2:213-23. [PubMed]
- Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr 2004;134:3463S-71S. [PubMed]
- Lointier P, Wargovich MJ, Saez S, et al. The role of vitamin D3 in the proliferation of a human colon cancer cell line in vitro. Anticancer Res 1987;7:817-21. [PubMed]
- Glinghammar B, Rafter J. Carcinogenesis in the colon: interaction between luminal factors and genetic factors. Eur J Cancer Prev 1999;8 Suppl 1:S87-94. [PubMed]
- Shabahang M, Buras RR, Davoodi F, et al. Growth inhibition of HT-29 human colon cancer cells by analogues of 1,25-dihydroxyvitamin D3. Cancer Res 1994;54:4057-64. [PubMed]
- Thomas MG, Tebbutt S, Williamson RC. Vitamin D and its metabolites inhibit cell proliferation in human rectal mucosa and a colon cancer cell line. Gut 1992;33:1660-3. [PubMed]
- Buras RR, Schumaker LM, Davoodi F, et al. Vitamin D receptors in breast cancer cells. Breast Cancer Res Treat 1994;31:191-202. [PubMed]
- Campbell MJ, Reddy GS, Koeffler HP. Vitamin D3 analogs and their 24-oxo metabolites equally inhibit clonal proliferation of a variety of cancer cells but have differing molecular effects. J Cell Biochem 1997;66:413-25. [PubMed]
- de Vos S, Holden S, Heber D, et al. Effects of potent vitamin D3 analogs on clonal proliferation of human prostate cancer cell lines. Prostate 1997;31:77-83. [PubMed]


