Obesity, a condition characterised by chronic hyperinsulinemia,
is a firmly established risk factor for incident
colon cancer (
1). With plausible biological explanations,
consistency of association, long durations between
anthropometric measurements (typically body mass
index, BMI) and cancer occurrence, and dose-effect with
increasing BMI, these associations are probably causal (
2).
Given this association, it is tempting to extrapolate that
increased body adiposity, and the inevitable concomitant
increased ‘insulin milieu’ at a tissue level, obesity may also
be associated with adverse treatment outcome, including
resistance to chemotherapies. Where BMI is determined
at baseline in prospective cohorts, obesity is certainly
associated with increased colon cancer-related mortality
(
3), but it is unclear at what steps on the cancer pathway,
excess adiposity exerts its inf luence. In patients with the
diagnosis of colon cancer undergoing 5-f luorouracilbased
adjuvant chemotherapy, pooled analysis from seven
randomized trials (n=4381) suggests that obesity is an
independent prognosticator (
4). However, there are caveats
– thus, among men with colon cancer, the significant
increased hazard ratio for overall survival is limited to
patients with BMI values greater than 35 kg/m
2; while
in women, reduced overall survival is limited to patients
with BMI values between 30 and 35 kg/m
2. The influence
of obesity in the setting of chemotherapy for metastatic colon cancer has been less extensively studied – although
one small non-randomized French series suggests that
surrogates of adiposity are not associated with oncological
outcome where conventional combined chemotherapy
is administered in metastatic colorectal cancer, but
increased visceral fat may predict for a reduced response to
bevacizumab-based therapies in the metastatic setting (
5).
In this issue of the journal, the study reported by Chen
and colleagues (
6) adds an interesting new dimension.
Using the HT29 colon cancer cell line, the authors show
that the addition of high-dose insulin in the presence
of oxaliplatin was associated with Akt activation and
chemoresistance, effects which were reversed by the
use of a PI3K inhibitor. The reductionist approach and
simplicity of the preclinical experiments renders these data
preliminary but certainly thought provoking. Furthermore,
given the mixed clinical observations summarized in the
opening paragraph, the reader may well ask, are these
findings clinically relevant? The answer is simple at one
level – obesity is a heterogeneous condition – and complex
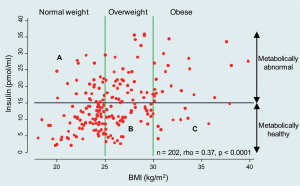
at many more levels. It is well known that serum insulin
levels increase with increasing BMI, but despite this good
correlation, as shown in Figure 1, there is wide variability.
Increasingly, the metabolic literature recognizes that
obesity may be dichotomized into metabolically benign
and malign states defined by criteria of insulin resistance,
subclinical inflammation and dyslipidemia. Based on recent
NHANES data, 23.5% of normal-weight US adults are
metabolically abnormal, whereas 51.3% of overweight adults
and 31.7% of obese adults are metabolically healthy (
7).
High circulating levels of insulin may prevail in both normal
weight and obese individuals and in turn, as depicted by
Chen and colleagues (
6), insulin may be pro-tumorigenic
either directly via the insulin receptor and insulin-like
growth factor I receptor (IGF-IR), or indirectly through
changes in the IGF-binding protein balance favoring IGFIR
activation. When one takes these into consideration, it is perhaps not surprising that BMI and other anthropometric
surrogates may not be ideal predictors of cancer treatment
and outcome. Further complexity is gleamed by the recent
recognition that the metabolically abnormal status of an
individual is more strongly driven by fatty liver changes
(non-alcoholic steatohepatitis, NASH) rather than by, as
conventionally believed, visceral (central) fat (
8).
Combinational oxaliplatin is now widely used in the
treatment of metastatic colorectal cancer, and in many
cases, the metastatic disease occurs in the liver. Initial
responses are good (greater than 50%) but the development
of chemoresistance is almost inevitable. Pulling together
the various new insights into insulin resistance and the
importance of fat distribution in the liver, the clinical
importance of the ‘insulin milieu’ and chemotherapy
becomes clearer. Excess liver fat (present in 20 to 25 % of
the population) is an important driver of insulin resistance
and hyperinsulinemia; while at the same time, NASH
may be associated with a peri-tumor environment rich in
pro-inflammatory factors and cytokines, favoring tumour
progression (
9).
The present study has defined the need for additional
exploration of the role of insulin and chemoresistance
in colon cancer. However, going forward, a number of
critical issues will need to be addressed if answers are to
be found. These include: a) consideration of the insulin
concentrations examined, typical molar concentrations
for in vitro experimentation range between 15 and 40 nM,
corresponding to the supra-physiological ranges depicted
in Figure 1, rather than 1000 nM used in the current study;
and b) the in vitro models employed, as chemoresistance
in in vitro models generally take several weeks to develop. Other colon cancer lines and chemotherapy agents need to
be explored.
Evaluating the effects of chronic insulin administration
on the PI3K/Akt pathway does indeed seem to be a worthy
pursuit. However, the cellular actions of insulin are likely to
be pleotropic and the endpoints of the PI3K/Akt pathway
extend beyond cell growth and apoptosis. Furthermore,
small-molecule inhibitors used to assess the physiological
roles of these enzymes should be cautiously interpreted,
and specifically for PI3K inhibition, PI-103 is now the
recommended in vitro tool, with superior specificity over
LY294002 (as used in the present study), and rapamycin,
a specific inhibitor of TORC1, should be used in parallel
to check whether any observed effects of PI-103 result
from TORC1 inhibition (
10). Finally, baseline mutational
profiling of the cell lines of interest should be considered.
HT29 cells are PI3K mutant, and as PIK3CA mutations
lead to increased basal phosphatidylinositol-3-kinase
activity, it is tempting to speculate that insulin signalling
is constitutive in these cells. However, our laboratory
has shown no distinguishing differences in cell growth
properties among cells carrying PIK3CA mutations from
a panel of commonly used colon cancer cell lines under
basal culture conditions (
11), but others have shown that
mutational activation of the PI3K/Akt pathway may be
essential for cellular growth under adverse conditions, and
for invasion (
12).
The paper of Chen and colleagues is timely, highlighting
the many complexities and challenges facing investigators
attempting to link clinical observations with biological
mechanisms in the field of obesity and cancer. To better
understand these complexities, there is a need for multi-disciplinary expertise to translate pre-clinical findings into
meaningful clinical benefit for our patients with colorectal
cancers.