
The targeted delivery of interleukin-12 to the carcinoembryonic antigen increases the intratumoral density of NK and CD8+ T cell in an immunocompetent mouse model of colorectal cancer
Introduction
Colorectal cancer represents the second leading cause of tumor-related deaths in the United States (1,2). Over the last decades, implementation of organized screening programs in the asymptomatic population have substantially decreased CRC incidence and overall mortality, by detecting cancer in its early stage (3,4). Nonetheless, ~22% of patients with CRC are still diagnosed with a metastatic condition (i.e., stage IV), or progress to late stage disease (5,6). Remarkable advances in the development of anti-cancer therapies have spawned multiple therapeutic strategies for the treatment of mCRC. First-line therapies involve the combination of 5-fluorouracil (FU)/leucovorin (LV) and oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) with monoclonal antibodies targeting the vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR) (7). Despite these advances, the 5-year survival rate of patients with mCRC continues to hover below 15% and new therapeutic strategies are therefore urgently needed (8).
In initial clinical studies including unselected groups of CRC patients, immune checkpoint inhibitors exhibited very limited anti-tumor activity. Closer evaluations underlined a small subset of patients experiencing long-term cancer remissions when treated with anti-PD1 antibodies (9). Genetic analyses revealed that these long-term survivors had highly mutated tumors (10,11), characterized by deficient mismatch repair (dMMR) and microsatellite instability-high (MSI-H) (8,12-14). These features are typically associated with cancer immunogenicity, presence of a substantial number of tumor-infiltrating lymphocytes (TILs) (15), and upregulation of immunological checkpoints. On the other hand, immune checkpoint inhibitors are not efficacious against MMR-proficient (pMMR) tumors (16), which account for the majority of the diagnosed mCRC. As these setbacks have been associated with immunologically poor tumors (8), strategies aiming at boosting cell-based immunity may be beneficial for this large proportion of patients with pMMR tumors.
In this study, we explored the antibody-based delivery of IL12 to the tumor microenvironment as a strategy to increase the intratumoral density of effector cells. We generated a novel fusion protein consisting of murine interleukin-12 sequentially fused with a peptidic linker to the Sm3E antibody in tandem diabody format (Sm3E-mIL12). Murine IL12 was used as surrogate therapeutic payload since human IL12 does not cross-react with the cognate murine IL12 receptor. The Sm3E antibody specifically targets the carcinoembryonic antigen (also called CEA or CEACAM5), a validated tumor-associated protein of gastrointestinal carcinomas (17,18). In healthy individuals, CEA’s expression is restricted to the apical surface of mature enterocytes (19), making the antigen virtually inaccessible from systemic circulation. However, when these epithelial cells turn malignant, the polarized expression of CEA breaks down, exposing the antigen to the vascular and lymphatic systems (20). As a result, serum levels of soluble CEA are routinely monitored to assess treatment response or disease recurrence in patients with CRC (21,22). Moreover, the selective accessibility in tumors of the membrane-bound CEA, make this protein an ideal target for monoclonal antibody-based therapies (23-25). In this study, we used a clinically translatable CEA-expressing tumor model (C51-CEA) previously established in our group, in order to investigate the anti-cancer potential of Sm3E-mIL12 in an immunocompetent setting (26).
The novel Sm3E-mIL12 immunocytokine retained a high binding affinity to the cognate antigen, and was potently active in vitro in terms of IFN-γ stimulation. Furthermore, the fusion protein was able to selectively localize in murine CEA subcutaneous tumors, while sparing healthy organs. Sm3E-mIL12 led to durable cancer eradications in 60% of the treated BALB/c mice, bearing established C51-CEA colon carcinomas. Microscopic analysis of the neoplastic masses revealed that the density of effector TILs was substantially increased after Sm3E-mIL12 treatment. The results of our study provide a rationale for the targeted delivery of IL12 for the treatment of pMMR colorectal cancer, possibly in combination with immune checkpoint inhibitors.
Material and methods
Cell lines, animals and tumor models
CHO-S (Invitrogen; CVCL_7183), and C51 colon carcinoma cells (kindly provided by Dr. M.P. Colombo, Department of Experimental Oncology, Istituto Nazionale Per Lo Studio E La Cura Dei Tumori, Milan, Italy) were expanded and stored as cryopreserved aliquots in liquid nitrogen. Cells were grown according to the manufacturer’s protocol and kept in culture for no longer than 14 passages. Authentication of the cell lines including post-freeze stability, growth properties and morphology, test for mycoplasma contamination, isoenzyme assay, and sterility were performed by the cell bank before shipment. C51 cells were stably transfected with CEA as previously described (26). All experiments were performed with mycoplasma-free cells. Seven to eight-week-old female BALB/c mice were obtained from Janvier; 2–4×106 cells (C51 colon carcinoma), were implanted subcutaneously in the left flank of the mice.
Cloning, expression and in vitro protein characterization.
The format chosen for Sm3E-mIL12 was inspired by previous work in our laboratory with F8 and L19 antibody derivatives (27). The sequence of the gene is reported in Figure S1. The insert was cloned into NheI/NotI of pcDNA3.1 (+) (Invitrogen), allowing the expression in mammalian cells. Sm3E-mIL12 was expressed using transient gene expression in Chinese Hamster Ovary (CHO) cells, using previously described procedures (28,29). The product was purified from the cell culture medium by affinity chromatography using a protein A affinity column and analyzed by SDS-PAGE, size exclusion chromatography (Superdex200 10/300GL, Healthcare), enzyme-linked immunosorbent assay (ELISA), flow cytometry (2L-Cytoflex, Beckman-Coulter), and surface plasmon resonance analysis (Biacore S200, GE Healthcare) on an CEA antigen-coated sensor chip (Sensor Chip SA, GE Healthcare; 10231984), following previously described protocols (26).
For the ELISA assay, soluble CEA was coated onto Nunc MaxiSorpTM wells (ThermoFisher, 44-2404-21). Binding was tested using Sm3E-mIL12, IgG2a(Sm3E) or KSF-mIL12 (KSF is an antibody specific to hen-egg lysozyme, used as negative control), which were subsequently detected with protein L-HRP (ThermoFisher, 32420). Positive binding was eventually confirmed through the reaction of the peroxidase with the TMB-Blotting Substrate Solution. Samples were analysed with a SpectraMax® Paradigm multimode detection platform (Molecular Devices) at 450 nm, and results are shown in terms of fold change in absorbance between Sm3E-mIL12 or IgG2a(Sm3E), as compared to the negative control.
Flow cytometry analysis was performed on C51.wt or C51-CEA cell. Proteins were labelled with fluorescein 5-isothiocyanate (FITC, F7250 Sigma) following the manufacturer’s protocol. Cells were resuspended at 3 mio/mL in FACS buffer (2% BSA, 2 mM EDTA in PBS) and membrane-bound CEA was detected using FITC-labelled Sm3E-mIL12 or KSF-mIL12 at 1 µg/mL. Data were analysed using a 2L-CytoFlex flow cytometer and subsequently with FlowJo 9 software suite (FlowJo LLC).
Protein stability assay
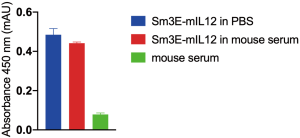
Sm3E-mIL12 was subjected to a protein stability assay in mouse serum (Sigma, M5905). The protein was incubated for 72 h at room temperature at 40 µg/mL (concentration at t0 upon intravenous injection, considering a dose of 60 µg). Sm3E-mIL12 diluted in PBS (40 µg/mL) was used as positive control, while mouse serum was as negative control for the experiment. Stability was analysed in terms of binding in ELISA, on CEA-coated Nunc MaxiSorpTM wells. The fusion protein was detected with goat anti-murine IL12 p70 (ThermoFisher) and rabbit anti-goat HRP (Dako) antibodies. The results are shown in Figure S2.
Bioactivity assay
Sm3E-mIL12, and recombinant mIL12 (BioLegend) were subjected to IFN-γ release assay. Lymphocytes were isolated from freshly dissected tumor-draining lymphnodes of saline treated 129/SvEv mice, bearing F9 teratocarcinoma. After red blood cell lysis, lymphocytes were resuspended at 3×106 cells/mL in RPMI-1640 (Gibco; 21875-034) supplemented with antibiotic-antimycotic (Gibco; 15240-062), 10% Fetal Bovine Serum (Gibco; 10270-106), 50 µM β-mercaptoethanol (Sigma Aldrich). 100 µL of the cell suspension was incubated for 4 days at 37 °C and 5% CO2 with a serial dilution of the IL12 derivatives. IFN-γ levels from cultured supernatants were analysed by enzyme-linked immunosorbent assay (BioLegend; 430804) following the manufacturer’s protocol.
Ex vivo biodistribution analysis
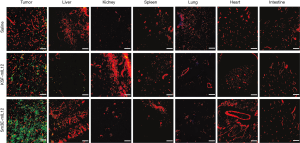
The tumor homing ability of Sm3E-mIL12 was assessed by an ex vivo biodistribution study; 200 µg of FITC-labelled Sm3E-mIL12 or KSF-mIL12 were injected into the lateral tail vein of BALB/c mice (Janvier) bearing C51-CEA tumors. Mice were sacrificed 24h after the injection. Organs were excised and embedded in cryoembedding medium (ThermoScientific) from which cryostat tissue sections (8–10 µm thickness) were made. FITC signal was amplified using rabbit anti-FITC (Bio-Rad, 4510-7804) and goat anti-rabbit AlexaFluor488 (Invitrogen, A1108). Signal amplification was required for the analysis with the wide field Axioskop2 mot plus microscope (Zeiss). For vascular staining, goat anti-CD31 (R&D System, AF3628) and anti-goat AlexaFluor594 (Invitrogen, A11058) antibodies were used. The quantification of tumor and organs uptake of the products, using Image J software, is depicted in Figure S3.

Therapy studies
Mice were monitored daily. Tumor volume was measured using a caliper (volume = length × width2 ×0.5). Mice were intravenously injected with 20, 45 and 60 µg of Sm3E-mIL12, and 60 µg KSF-mIL12, every second day for three times. The therapeutic agent was diluted in Phosphate Buffer Saline (PBS; Gibco). Animals were euthanized when tumors reached a maximum of 1,500 mm3 (n=5 mice per group).
Analysis of tumor-infiltrating lymphocytes (TILs)
To analyse TILs, mice were injected intravenously three times, every second day, with 60 µg Sm3E-mIL12, 60 µg KSF-mIL12 or saline. Treated mice were euthanised 24 h after the first and third injection. Tumors were excised and embedded in cryoembedding medium (ThermoScientific) and the corresponding cryostat tissue sections (8–10 µm thickness) were stained using the following primary antibodies: goat anti-CD31 (R&D System; AF3628), rabbit anti-Foxp3 (Invitrogen; 7000914), rat anti-NKp46 antibody (BioLegend; 137602), rabbit anti-CD4 (Sino Biological; 50134-R001), rabbit anti-CD8 (Sino Biological; 50389-R208). Primary antibodies were detected with donkey anti-rabbit AlexaFluor488 (Invitrogen; A11008) or donkey anti-rat AlexaFluor488 (Invitrogen; A21208) and donkey anti-goat AlexaFluor594 (Invitrogen; A21209) or donkey anti-goat AlexaFluor488 (Invitrogen; A11055). Slides were mounted with fluorescent mounting medium (Dako Agilent) and analyzed with Nikon Eclipse Ti-E fully-integrated, motorized inverted microscope (Tokyo, Japan).
Results
Cloning and characterization of the Sm3E-mIL12 fusion protein
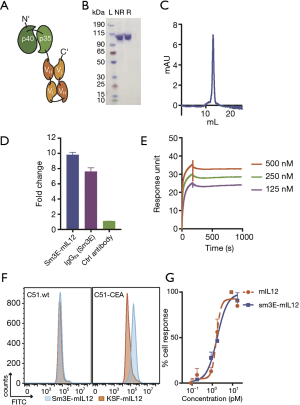
Figure 1A shows a schematic representation of the novel Sm3E-mIL12 immunocytokine. The p40 and p35 subunits of murine IL12 have been sequentially fused to the N’-terminal site of the Sm3E antibody, cloned in tandem diabody format. The product was purified through protein A affinity chromatography, and impurities were analysed by SDS-PAGE (Figure 1B) followed by gel filtration chromatography (Figure 1C). Sm3E-mIL12 bound efficiently to both soluble and membrane-bound CEA (C51-CEA), as evidenced by ELISA, Surface Plasmon Resonance and Flow Cytometry binding studies (Figure 1D,E,F). The ability of the fusion protein to induce IFN-γ production was confirmed in a cell-based assay using murine IL12 as reference, yielding with a EC50 values of 1.583 and 1.753 pM, respectively (Figure 1G).
Biodistribution study of FITC-labelled Sm3E-mIL12 and KSF-mIL12
The ability of Sm3E-mIL12 to localize at the tumor site was assessed through an in vivo biodistribution study in immunocompetent BALB/c mice, bearing C51-CEA lesions (saline treatment was used as negative control). 24 h after intravenous administration, the fusion proteins were detected ex vivo by immunofluorescent staining (Figure 2). While the two products were undetectable in healthy organs, the tumor uptake was substantially higher when IL12 was fused to the tumor-targeting antibody (i.e., Sm3E-mIL12) as compared to KSF-mIL12. Moreover, as evidenced by a protein stability assay, Sm3E-mIL12 retained binding to the cognate antigen after being incubated for 72 h in mouse serum (Figure S2).
Therapy experiments
In a preliminary dose escalation experiment conducted in BALB/c mice bearing C51-CEA subcutaneous tumors, Sm3E-mIL12 was well tolerated up to 60 µg, as evidenced by the absence of body weight loss (Figure 3A). Moreover, a cumulative dose of 180 µg exerted a potent anti-cancer cancer effect on small tumors. In a therapy study performed on mice carrying well established subcutaneous lesions, Sm3E-mIL12 exerted a potent tumor-growth inhibition leading to durable complete responses in 60% of the treated animals. By contrast, the untargeted IL12 (i.e., KSF-mIL12) did not show a comparable therapeutic performance (Figure 3B).
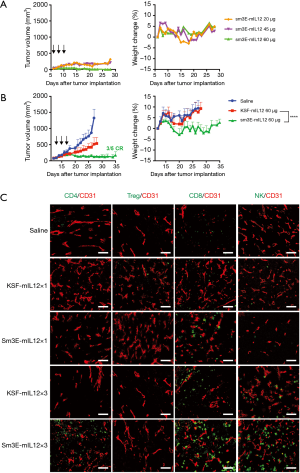
Microscopic analysis of tumor-infiltrating lymphocytes
In order to gain insights on the mechanism of action of Sm3E-mIL12, we analysed the immune cell infiltrate of C51-CEA tumors, excised 24 h after the first and last injection of the immunocytokine (i.e., 1 and 5 days after the first injection). A progressive infiltration of NK cells, CD4+ and CD8+ T cells within the tumor mass could be observed along with Sm3E-mIL12 treatment (Figure 3C). By contrast, the number of regulatory T cells remained unchanged.
Discussion
In this Communication, we have described the characterization of a novel immunocytokine consisting of murine interleukin-12 fused to the tumor-targeted Sm3E antibody (Sm3E-mIL12) (Figure 1). Sm3E is a humanized immunoglobulin with high binding affinity to both soluble and cell membrane-bound CEA (Kd =20 pM). Sm3E-mIL12 efficiently localized at the tumor site and not in healthy organs, thus potentially reducing systemic toxicity (Figure 2). Sm3E-mIL12 led to a complete tumor eradication 60% of the treated mice, while murine IL12 fused to KSF (a control antibody specific to hen egg lysozyme) induced only a transient and not significant inhibition of the tumor growth (Figure 3B). The anti-cancer activity of Sm3E-m-IL12 correlated with a progressive increase of CD4+, CD8+ T cells and NK cells within the tumor mass, consistent with the regression of the neoplastic lesions (Figure 3C). The increase of effector cells might derive from an expansion of pre-existent tumor-resident lymphocytes, or from newly infiltrating leukocytes attracted at the site of disease by chemokine gradients. Previous work of our group showed a dramatic elevation of IFN-inducible protein 10 (IP10) and of monokine-induced by IFNγ (MIG) in cancer lysates upon treatment with a tumor-targeted IL12 (30). Furthermore, depletion experiments have shown that the presence of IP10 and MIG is crucial, at least in mouse models, to achieve a robust T cell infiltration within the tumor mass (31).
CEA is a validated tumor-associated antigen discovered by Phil Gold and Samuel Freeman in 1965 (32). In healthy individuals, the expression of CEA is restricted to the apical surface of polarized epithelial cells across the gastrointestinal tract (21). Therefore, the membrane-bound antigen is not accessible to therapeutic proteins from systemic circulation. However, when the epithelial cells become malignant, CEA can be found around the whole cell surface as a result of tumor cells differentiation (21). The selective accessibility of CEA in cancer patients cancer has made it an ideal target for both imaging and therapeutic applications (24,25).
At present, systemic therapeutic strategies are not efficacious in controlling mCRC, except for a small subset of patients with highly mutated cancers (i.e., dMMR-MSI-H tumors) that respond well to immune checkpoint inhibitors (9). The high response rate to immunotherapy observed in this population has been correlated with the presence of a substantial number of TILs (33). However, the majority of mCRC have a pMMR phenotype (~85%) characterized by low immunogenicity, few TILs, and are consequently irresponsive to immunotherapy. It is now clear that the presence of pre-existent tumor-specific lymphocytes represents a crucial biomarker of responsiveness to anti-PD1 treatments (34-36). Beyond immune checkpoint inhibitors, bispecific antibodies are currently being investigated in pMMR CRC (37). Cibisatamab, a bispecific antibody which bridges CEA+ and CD3+ cells, showed promising anti-cancer activity in combination with Tecentriq® (atezolizumab) (NCT02650713) (38,39). In principle, an immunocytokines directed to a tumor cell membrane antigen may be equivalent to a bispecific entity, creating an immunological synapse between the cancer cell and suitable leukocytes expressing the cognate cytokine receptors (e.g., T and NK cells).
IL12 is a strong modulator of the immune system which boosts the activity of T cells and NK cells. However, recombinant IL12 induces life threatening side-effects at really low doses (i.e., 500 ng/kg in human patients), thus preventing dose escalation to therapeutically effective regimens (40,41). The fusion of IL12 to the tumor-targeting Sm3E antibody provides a strategy to reduce exposure of the pro-inflammatory cytokine to healthy organs, while enhancing activity at the site of disease. In this study, the antibody-based delivery of IL12 to the neoplastic mass has shown to strongly enhance the number of TILs. Taken together, our data suggest that Sm3E-IL12 may be a suitable product to combine with immune checkpoint inhibitors for the treatment of pMMR which lack of effector T cells.
Acknowledgments
This work was supported by Eidgenössische Technische Hochschule Zürich.
Funding: This work was supported by the Swiss National Science Foundation (Grant Nr. 310030_182003/1) and by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement 670603).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2020.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (license number 04/2018) granted by the Veterinäramt des Kantons Zürich, Switzerland, in compliance with the Swiss Animal Protection Act (TSchG) and the Swiss Animal Protection Ordinance (TSchV).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med 2017;65:311-5. [Crossref] [PubMed]
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009;22:191-7. [Crossref] [PubMed]
- Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol 2017;23:5086-96. [Crossref] [PubMed]
- Garborg K, Holme O, Loberg M, et al. Current status of screening for colorectal cancer. Ann Oncol 2013;24:1963-72. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1-9. [Crossref] [PubMed]
- Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019;16:361-75. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69. [Crossref] [PubMed]
- Smyrk TC, Watson P, Kaul K, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2001;91:2417-22. [Crossref] [PubMed]
- Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202-6. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485-90. [Crossref] [PubMed]
- Nap M, Mollgard K, Burtin P, et al. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol 1988;9:145-53. [Crossref] [PubMed]
- Graff CP, Chester K, Begent R, et al. Directed evolution of an anti-carcinoembryonic antigen scFv with a 4-day monovalent dissociation half-time at 37°C. Protein Eng Des Sel 2004;17:293-304. [Crossref] [PubMed]
- Tiernan JP, Perry SL, Verghese ET, et al. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br J Cancer 2013;108:662-7. [Crossref] [PubMed]
- Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011;19:620-6. [Crossref] [PubMed]
- Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 1999;9:67-81. [Crossref] [PubMed]
- Chester KA, Begent RH. Circulating immune complexes (CIC), carcinoembryonic antigen (CEA) and CIC containing CEA as markers for colorectal cancer. Clin Exp Immunol 1984;58:685-93. [PubMed]
- Boonstra MC, Tolner B, Schaafsma BE, et al. Preclinical evaluation of a novel CEA-targeting near-infrared fluorescent tracer delineating colorectal and pancreatic tumors. Int J Cancer 2015;137:1910-20. [Crossref] [PubMed]
- Willkomm P, Bender H, Bangard M, et al. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of the recurrence of colorectal carcinoma. J Nucl Med 2000;41:1657-63. [PubMed]
- Mayer A, Tsiompanou E, O’Malley D, et al. Radioimmunoguided Surgery in Colorectal Cancer Using a Genetically Engineered Anti-CEA Single-Chain Fv Antibody. Clin Cancer Res 2000;6:1711. [PubMed]
- Bajic D, Chester KA, Neri D. An antibody-tumor necrosis factor fusion protein that synergizes with oxaliplatin for treatment of colorectal cancer. bioRxiv 2019.698563.
- Pasche N, Wulhfard S, Pretto F, et al. The Antibody-Based Delivery of Interleukin-12 to the Tumor Neovasculature Eradicates Murine Models of Cancer in Combination with Paclitaxel. Clin Cancer Res 2012;18:4092-103. [Crossref] [PubMed]
- Pasche N, Woytschak J, Wulhfard S, et al. Cloning and characterization of novel tumor-targeting immunocytokines based on murine IL7. J Biotechnol 2011;154:84-92. [Crossref] [PubMed]
- Rajendra Y, Kiseljak D, Baldi L, et al. A simple high-yielding process for transient gene expression in CHO cells. J Biotechnol 2011;153:22-6. [Crossref] [PubMed]
- Puca E, Probst P, Stringhini M, et al. The antibody-based delivery of interleukin-12 to solid tumors boosts NK and CD8+ T cell activity and synergizes with immune checkpoint inhibitors. Int J Cancer 2020;146:2518-30. [Crossref] [PubMed]
- Tannenbaum CS, Tubbs R, Armstrong D, et al. The CXC Chemokines IP-10 and Mig Are Necessary for IL-12-Mediated Regression of the Mouse RENCA Tumor. J Immunol 1998;161:927. [PubMed]
- Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965;122:467-81. [Crossref] [PubMed]
- Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43-51. [Crossref] [PubMed]
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. [Crossref] [PubMed]
- Overman MJ, Lonardi S, Wong KYM, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol 2018;36:773-9. [Crossref] [PubMed]
- Andre T, Lonardi S, Wong M, et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142. J Clin Oncol 2018;36:553. [Crossref]
- Oberst MD, Fuhrmann S, Mulgrew K, et al. CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. mAbs 2014;6:1571-84. [Crossref] [PubMed]
- Bacac M, Klein C, Umana P. CEA TCB: A novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunology 2016;5:e1203498. [Crossref] [PubMed]
- Tabernero J, Melero I, Ros W, et al. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). J Clin Oncol 2017;35:3002. [Crossref]
- Portielje JEA, Kruit WHJ, Schuler M, et al. Phase I Study of Subcutaneously Administered Recombinant Human Interleukin 12 in Patients with Advanced Renal Cell Cancer. Clin Cancer Res 1999;5:3983-9. [PubMed]
- Leonard JP, Sherman ML, Fisher GL, et al. Effects of Single-Dose Interleukin-12 Exposure on Interleukin-12–Associated Toxicity and Interferon-γ Production. Blood 1997;90:2541-8. [PubMed]



