
Critical evaluation of quality of hepatopancreatic surgery in a medium-volume center in Finland using the Accordion Severity Grading System and the Postoperative Morbidity Index
Introduction
Hepatopancreatic resections constitute a field of surgery prone to complications (1-3). These procedures demand high acuity from the surgeons, as well as from the entire process, from administration to discharge and during the following weeks. Benefits of high annual center and surgeon volume have led to centralization of major surgical operations to achieve the best possible results (2,4,5).
The Postoperative morbidity index (PMI) is a quantitative severity weighting system; it uses the modified Accordion Severity System (MASGS) to grade the severity of complications gathered from American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) complication data, and then weights each complication (6). This quantitative measure was first applied to several surgical procedures such as appendectomy, laparoscopic colectomy, and hepatectomy in 2011 (7), and to distal pancreatectomies in 2013 (8). In 2015, a quantitative benchmark for morbidity in pancreaticoduodenectomy (PD) was established using PMI (1), and the complication burden of 64 total pancreatectomies was described using the same methods (9). The established scoring system enables the assessment and comparison of surgical results between institutions, highlights targets for improvement, and may further determine benchmark complication cut-offs for results to be achieved (1,2).
Pancreatic and hepatic surgery, especially major procedures including PD (1) or major (≥3 segments) resection, carry high complication burden even in low-risk patients (1-3,10). Distal pancreatic resection and minor (≤2 segments) hepatic resection are less prone to severe complication (3). There is high variability in the number of complications and survival between the best and worst performing major surgical hospitals (1-3), indicating the need for routine quantitative benchmarking in all hospitals in which major surgery is performed in order to improve performance and reduce variation in outcomes.
The aim of this study was to compare different hepatopancreatic procedures by means of a quantitative scoring system in a medium-volume center. The complication profile and burden were compared with benchmark studies published earlier (1,2,9). The secondary aim was to critically evaluate the rationale of major surgery performed in our center and targets for improvement.
Methods
Study design
This was a retrospective cohort study. Included patients had received either pancreatic or liver resection in 2000–2017 at Central Finland Central Hospital. Data about complications were collected prospectively and re-reviewed retrospectively by another researcher. The histopathological diagnoses included mainly malignant diseases, such as ductal adenocarcinoma of pancreas, colorectal liver metastases, and hepatocellular carcinoma.
Procedural data
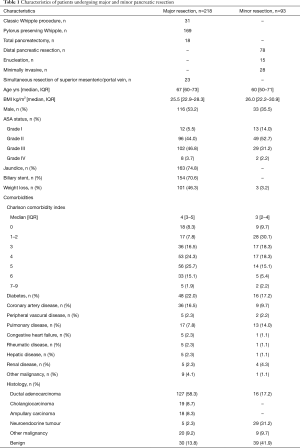
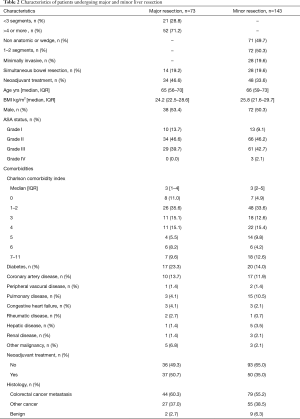
Pancreaticoduodenectomies and total pancreatectomies are considered major pancreatic resections; distal pancreatic resections are considered minor resections. Similarly, liver resections comprising three or more segments of the liver are considered major resections, and the rest are considered minor resections. All major resections were open operations; 19.6% of minor resections were performed with a minimally invasive approach. The standard Whipple technique was used in 15.5% and pylorus-preserving technique in 84.5% of pancreaticoduodenectomies. Of major liver resections, 71.2% were hemihepatectomies or extended hemihepatectomies. Simultaneous bowel resection was performed in 19.2% of major and 19.6% of minor liver resections (Tables 1,2).
Full table
Full table
Assignment of complication severity grades, complication burden, and PMI
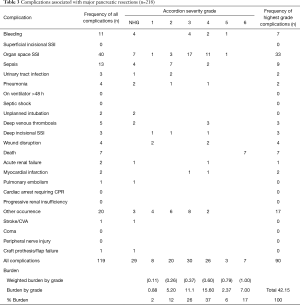
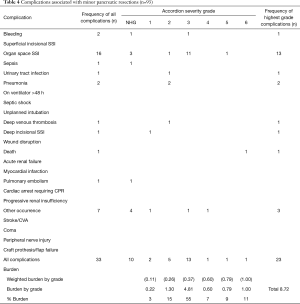
To enable comparison of our results with the results published by others, we scored the complications as described by Vollmer et al. in the benchmark study (1). Therefore, for each patient who developed a postoperative complication within 30 days, a complication grade from 1 to 6 was assigned according to the rules of the MASGS (6), which is nearly analogous with the commonly used Clavien-Dindo classification (11). When a patient developed more than 1 complication, they were assigned the grade of the most severe complication, referred to as the highest grade complication. When other NSQIP complications of lesser severity occurred in the same patient, they were referred to as not highest grade (NHG) complications. The number and severity of complications based on MASGS and NSQIP are presented separately for major and minor pancreatic and hepatic procedures (Tables 3-6).
Full table
Full table
Full table
Full table
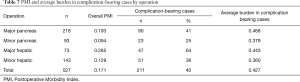
After grading highest grade complications from 1 to 6 according to the MASGS, the complications were weighted using the previously derived severity grade-related utility weights (Tables 3-6). The PMI is calculated by dividing the burden of all highest grade complications by the number of patients in the study, thus providing a population-level measure for morbidity (Table 7). To be clear, PMI could vary between zero (no patient suffered a complication) and 1.0 (all patients died in 30 days).
Full table
Assignment of non-NSQIP complications
For patients who underwent PD (n=200), the rates of postoperative pancreatic fistula (POPF), biliary leaks, and delayed gastric emptying (DGE) were collected prospectively. POPFs were classified according to International Study Group of Pancreatic Fistula (ISGPF) updated definition as grade B or grade C fistula (12,13). Biliary leakage was graded according to International Study Group of Liver Surgery (ISGLS) grading system (14). DGE was classified according the International Study Group of Pancreatic Surgery (15). Postoperative hepatic dysfunction was defined as prolonged hyperbilirubinemia (unrelated to obstruction or leak), ascites, and/or encephalopathy.
The study was approved by the Central Finland Hospital District. Because of the observational nature of the study, patient informant consent was not required.
Results
Baseline characteristics of the study population
In all, 527 consecutive patients that had undergone pancreatic or liver resection were included in this study. A total of 218 major and 93 minor pancreatic resections were performed during the study period. The histological diagnosis was pancreatic ductal adenocarcinoma in 127 (58.3%) and 16 (17.2%), other cancer in 62 (28.9%) and 38 (40.9%), and benign disease in 30 (13.8%) and 39 (41.9%) patients, respectively (Table 1).
Seventy-three major and 143 minor hepatic resections were performed. The histological diagnosis was colorectal cancer metastasis in 44 (60.3%) and 79 (55.2%), other cancer in 37.0% and 38.5%, and benign disease in 2.7% and 6.3% of patients, respectively (Table 2). Simultaneous bowel resection was performed in 19.2% and 19.6% of patients, respectively (Table 2).
The mean annual number of pancreatic resections was 11 between 2000–2007 and increased to 22 from 2008–2017. Respective figures in liver surgery were 6 and 17. Patient characteristics, including comorbidities using the Charlson Comorbidity Index (CCI), are presented in Tables 1,2.
Major pancreatic resections
The frequency of NSQIP complications by severity grade is shown in Table 3. In all, 41.1% of patients suffered at least one NSQIP complication, and 16.9% had more than one complication. Major complications (MASGS 4-6) occurred in 13.3% of cases. The overall number of complications was 171. The most frequently encountered complications were organ space surgical site infection (n=40) and other occurrence (n=20). Other occurrence consisted of complications like chylus leak, arterial thrombosis, arrythmia, and postoperative ileus.
Minor pancreatic resections
The frequency of NSQIP complications by severity grade is shown in Table 4. In all, 24.7% of patients suffered at least one NSQIP complication, and 9.8% had more than one complication. Major complications (MASGS 4-6) occurred in 3.3% of cases. The overall number of complications was 33. The most frequently encountered complication was organ space surgical site infection (n=16).
Major liver resections
The frequency of NSQIP complications by severity grade is shown in Table 5. In all, 64.4% of patients suffered at least one NSQIP complication, and 17.8% had more than one complication. Major complications (MASGS 4-6) occurred in 19.2% of cases. The overall number of complications was 80. The most frequently encountered complications were organ space surgical site infections (n=11), pneumonia (n=11), and bleeding (n=7).
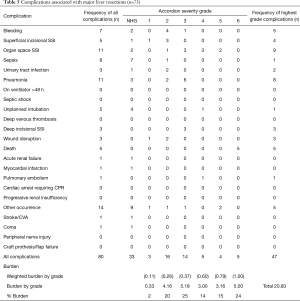
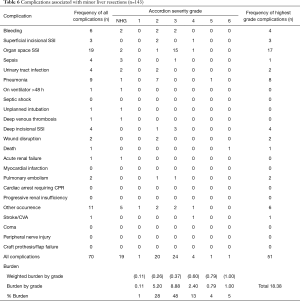
Minor liver resections
The frequency of NSQIP complications by severity grade is shown in Table 6. In all, 35.7% of patients suffered at least one NSQIP complication, and 9.8% had more than one complication. Major complications (MASGS 4-6) occurred in 6.3% of cases. The overall number of complications was 70. The most frequently encountered complications were organ space surgical site infections (n=19) and pneumonia (n=9).
Burden of complications and Postoperative Morbidity Index
The weighted burden of NSQIP complications separately for major and minor pancreatic and hepatic procedures is presented in the end of Tables 3-6. The PMI for all 527 patients was 0.177, as shown in Table 7. The PMI for patients who received major pancreatic resection was 0.192, and the PMI for patients receiving minor pancreatic resection, major liver resection, and minor liver resection was 0.094, 0.285 and 0.129, respectively.
Non-NSQIP complications
We also collected procedure-specific outcomes, not accrued by NSQIP methodology, to enable comparison of our results with those of other hospitals.
For patients who received PD (n=200), the POPF rates were 6.5% for grade B and 5.5% for grade C. The biliary leak rates were 1.0% for grade A leakage, 2.5% for grade B leakage and 0.5% for grade C leakage. The DGE rates were 2.8% for grade A, 15.6% for grade B and 3.7% for grade C.
For patients who received liver resection, major surgical site bleeding occurred in 5 (2.3%) and biloma occurred in 17 (7.9%) patients. Postoperative hepatic dysfunction occurred in 5 (2.3%) patients. In patients undergoing major hepatic resection, median (IQR) laboratory values at postoperative day 3 were as follows: alkaline phosphatase 98 [57–176], alanine aminotransferase 350 [274–546], bilirubin 28 [17–46], ammonia 43 [31–64], and INR 1.6 [1.5–1.9]. Preoperative albumin level was 37 [36–40]. Respectively, values in patients with minor hepatic resection were: alkaline phosphatase 79 [57–130], alanine aminotransferase 299 [135–527], bilirubin 14 [10–22], ammonia 34 [19–43], and INR 1.4 [1.2–1.6]. Preoperative albumin level was 38 [35–40].
The median [IQR] duration of stay was 12 [8–17] days for major pancreatic resections, 7 [6–10] days for minor pancreatic resections, 9 [7–14] days for major liver resections, and 7 [6–11] days for minor liver resections.
The 30-day readmission rate was 9.6% for patients with major pancreatic resections, 4.3% for those with minor pancreatic resections, 17.8% for those with major liver resections, and 7.0% for those with minor liver resections. The 30-day mortality rates were 3.2% and 1.1% in major and minor pancreatic resections, respectively, and 6.8% and 0.7% in major and minor liver resections, respectively. The 90-day mortality rates were 3.7% and 1.1% in major and minor pancreatic resections, respectively, and 8.2% and 0.7% in major and minor liver resections, respectively.
Complications over time
To compare differences in complication rates over time we divided data into two equal sized groups (2000–2010, 2011–2017). In major pancreatic resections overall complication rates in former and latter time period were 41.5% and 41.1%, P=0.957. Major complications occurred in 10.6% and 15.3%, P=0.313, respectively. After minor pancreatic resections overall complication rates were 20.4% and 29.5%, P=0.308, and major complication rates 2.0% and 4.5%, P=0.495, respectively.
In major liver resections overall complication rates in former and latter time period were 72.7% and 45.0%, P=0.017. Major complications occurred in 24.2% and 15.0%, P=0.318, respectively. After minor liver resections overall complication rates were 32.2% and 40.5%, P=0.313, and major complication rates 8.5% and 4.8%, P=0.368, respectively.
Discussion
This study is, to our knowledge, the first to compare different hepatopancreatic procedures using a quantitative severity weighting system. PMI was found informative, giving a simple value that includes information about both the number and the severity of complications for comparison with other procedures or institutions. In general, the quality of surgery in our center was in line with that reported earlier in the literature (16). However, the complication burden level measured with PMI in the benchmark studies was not fully achieved. Postoperative mortality was in line with that reported earlier in the literature (17-19).
Particularly, the PMI of 0.192 for major pancreatic resections was relatively high compared with the results of the institutions involved in Vollmer’s benchmark study (1) or the results Strasberg et al. reported in 2011 (7). Vollmer et al. reported PMI varying between 0.097–0.239 depending on institution, whereas Strasberg et al. reported PMI 0.150 for a small sample of patients who underwent PD. In the present study, the proportion of complications “other occurrence” was significantly higher (16.8%) compared to papers mentioned earlier. “Other occurrence” consisted of complications like chylus leak, arterial thrombosis, arrythmia, and ileus in the present study. The low proportion of “other occurrence” (0.2%) in the benchmark study by Vollmer et al. indicates the possibility that such complications were ignored when focusing on NSQIP. Moreover, 5% of pancreaticoduodenectomies in the present study were performed with extended lymphadenectomy, a procedure that is known to be associated with higher complication rates (20). Nevertheless, the rate of pancreatic-specific non-NSQIP complications were congruent with rates reported earlier in the literature (1,21). Similarly, the overall morbidity of 41.1% in our study is equal to or lower than rates reported earlier in literature (10,16). The postoperative morbidity of patients who underwent minor pancreatic resection was low or equal when compared with the results reported earlier (8,22,23).
Strasberg et al. reported PMI 0.145 in 52 major hepatic resections (7), which is significantly lower than PMI 0.285 in our study. However, simultaneous bowel resection was performed in 19% of patients in our study, which is associated with higher complication rates and more serious complications (7,24). Furthermore, variations have been reported in complication rates after liver surgery in liver donors and recipients of liver transplant, with overall complications ranging from 27% to 80% and major complications ranging from 6% to 42% (2,25). Liver donors are, however, young and healthy people; in contrast, patients undergoing liver resection for malignant disease tend to be older, have comorbid conditions, and are often receiving neoadjuvant chemotherapy. Our postoperative mortality rate (2.8%) after liver surgery was in agreement with the range of 1.8–5.6% reported in population-based studies from Sweden and the United States, with ratios of major and minor resections very similar to our study (26). Furthermore, population-based studies (such as ours) tend to have higher morbidity and mortality rates than single-center reports, possibly due to selective reporting (26).
Textbook outcomes, defined as no postoperative surgical complications, prolonged hospital stays, or readmissions, have been recently reported for minor and major pancreatic and liver resections classified similarly to those in our study (3). In the study by Merath et al., textbook outcome was not achieved in 75.3% of patients who underwent major pancreatic resection and in 52.2% who underwent minor pancreatic resection. Respective values among patients with major and minor liver resection were 66.7% and 53.2% (3). This corresponds to or exceeds our overall complication values of 41.1%, 24.7%, 64.4%, and 35.7%, respectively. The 30-day readmission rate of patients who underwent major pancreatic resection was 9.6% in the present study. This is lower than in the benchmark study (1), but can be partly explained by longer hospital stays in our study. In patients who underwent major and minor liver resection, the median hospital stays were 9 and 7 days, respectively, and 30-day readmission rates were 17.8% and 7%, respectively. These numbers are comparable with registry data on hepatopancreatobiliary surgery from the United States, where 7-day hospital stays and 17% readmission rates were reported (27). In all, 30.1% of minor pancreatic resections were done in a minimally invasive manner, a notably high rate taking into account that the first laparoscopic pancreatic resection was done at our center in 2007. We observed no major changes in complication rates over time, except some improvements in major liver resections.
Organ space surgical site infections were the most common complications in both pancreatic and liver surgeries. Infected pancreatic fistula is the obvious cause for the high number of organ space surgical site infections after pancreatic resections, and simultaneous bowel and liver surgery after liver resections. No single complication type differed significantly from previous reports, and therefore an easy solution to improve postoperative morbidity could not be found. Both major pancreatic and liver resections were associated with heavy burdens of complication, showing PMIs twice as high as in minor resections.
Our results should be interpreted with some caution, however. The major weakness of this study is the relatively small number of patients compared to large international patient series. The quantitative complication scoring system enables comparison between different operations and centers with exact thresholds for what actually constitutes a complication, but it also lacks data on procedure-specific complications (such as POPF and DGE after pancreatic surgery, and biloma formation and liver failure after hepatic surgery). These limitations have been recognized previously (28). For this reason, we reported procedure-specific complications separately as non-NSQIP complications, according to Vollmer et al. (1). The strengths of this study include prospective data collection, double checking of the hospital records by another researcher not responsible of treating these patients, and complete follow-up data. Moreover, all patients from a single geographic area were included in the study, making selection bias unlikely and illustrating the real-life situation. Treatment of late complications is conducted in our center, which is the only hospital in the area.
In conclusion, PMI appears to be an informative method to monitor outcomes in pancreatic and hepatic surgery enabling demonstrative comparison between various procedures and institutions. Our results are comparable with previous population- and registry-based results, but improvements are needed to achieve reported benchmark levels.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2020.04.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Central Finland Hospital District. Because of the observational nature of the study, patient informant consent was not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vollmer CM Jr, Lewis RS, Hall BL, et al. Establishing a quantitative benchmark for morbidity in pancreatoduodenectomy using ACS-NSQIP, the Accordion Severity Grading System, and the Postoperative Morbidity Index. Ann Surg 2015;261:527-36. [Crossref] [PubMed]
- Rössler F, Sapisochin G, Song G, et al. Defining Benchmarks for Major Liver Surgery: A multicenter Analysis of 5202 Living Liver Donors. Ann Surg 2016;264:492-500. [Crossref] [PubMed]
- Merath K, Chen Q, Bagante F, et al. Textbook Outcomes Among Medicare Patients Undergoing Hepatopancreatic Surgery. Ann Surg 2020;271:1116-23. [PubMed]
- Ahola R, Siiki A, Vasama K, et al. Effect of centralization on long-term survival after resection of pancreatic ductal adenocarcinoma. Br J Surg 2017;104:1532-8. [Crossref] [PubMed]
- Macedo FIB, Jayanthi P, Mowzoon M, et al. The Impact of Surgeon Volume on Outcomes After Pancreaticoduodenectomy: a Meta-analysis. J Gastrointest Surg 2017;21:1723-31. [Crossref] [PubMed]
- Porembka MR, Hall BL, Hirbe M, et al. Quantitative weighting of postoperative complications based on the accordion severity grading system: demonstration of potential impact using the american college of surgeons national surgical quality improvement program. J Am Coll Surg 2010;210:286-98. [Crossref] [PubMed]
- Strasberg SM, Hall BL. Postoperative morbidity index: a quantitative measure of severity of postoperative complications. J Am Coll Surg 2011;213:616-26. [Crossref] [PubMed]
- Lee MK 4th, Lewis RS, Strasberg SM, et al. Defining the post-operative morbidity index for distal pancreatectomy. HPB (Oxford) 2014;16:915-23. [Crossref] [PubMed]
- Datta J, Lewis RSJ, Strasberg SM, et al. Quantifying the burden of complications following total pancreatectomy using the postoperative morbidity index: a multi-institutional perspective. J Gastrointest Surg 2015;19:506-15. [Crossref] [PubMed]
- Leichtle SW, Kaoutzanis C, Mouawad NJ, et al. Classic Whipple versus pylorus-preserving pancreaticoduodenectomy in the ACS NSQIP. J Surg Res 2013;183:170-6. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584-91. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Malleo G, Crippa S, Butturini G, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB (Oxford) 2010;12:610-8. [Crossref] [PubMed]
- Diener MK, Knaebel HP, Heukaufer C, et al. A Systematic Review and Meta-analysis of Pylorus-preserving Versus Classical Pancreaticoduodenectomy for Surgical Treatment of Periampullary and Pancreatic Carcinoma. Ann Surg 2007;245:187-200. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol 2016;113:188-93. [Crossref] [PubMed]
- Seppänen H, Juuti A, Mustonen H, et al. The Results of Pancreatic Resections and Long-Term Survival for Pancreatic Ductal Adenocarcinoma: A Single-Institution Experience. Scand J Surg 2017;106:54-61. [Crossref] [PubMed]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002;236:355-66; discussion 366-8. [Crossref] [PubMed]
- Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore) 2017;96:e6858. [Crossref] [PubMed]
- Riviere D, Gurusamy KS, Kooby DA, et al. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane database Syst Rev 2016;4:CD011391. [PubMed]
- Madureira FAV, Gres P, Vasques RR, et al. Predictive factors of morbidity in distal pancreatic resections. Rev Col Bras Cir 2012;39:496-501. [Crossref] [PubMed]
- Ratti F, Catena M, Di Palo S, et al. Impact of totally laparoscopic combined management of colorectal cancer with synchronous hepatic metastases on severity of complications: a propensity-score-based analysis. Surg Endosc 2016;30:4934-45. [Crossref] [PubMed]
- Muller X, Marcon F, Sapisochin G, et al. Defining Benchmarks in Liver Transplantation: A Multicenter Outcome Analysis Determining Best Achievable Results. Ann Surg 2018;267:419-25. [Crossref] [PubMed]
- Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg 2008;12:842-51. [Crossref] [PubMed]
- Merath K, Bagante F, Chen Q, et al. The Impact of Discharge Timing on Readmission Following Hepatopancreatobiliary Surgery: a Nationwide Readmission Database Analysis. J Gastrointest Surg 2018;22:1538-48. [Crossref] [PubMed]
- Epelboym I, Gawlas I, Lee JA, et al. Limitations of ACS-NSQIP in reporting complications for patients undergoing pancreatectomy: underscoring the need for a pancreas-specific module. World J Surg 2014;38:1461-7. [Crossref] [PubMed]


