Management of periampullary adenocarcinoma by pancreaticoduodenectomy at a regional teaching hospital
Introduction
Periampullary adenocarcinoma (PA) is a term encompassing four epithelial malignancies: carcinoma of the head of the pancreas (HOP), duodenal carcinoma, ampullary carcinoma and cholangiocarcinoma involving the distal common bile duct (1,2). Because of the similarities in location and natural history among these malignancies, and because a precise, preoperative diagnosis is sometimes elusive, these tumors are approached in the same way. In the setting of non-metastatic disease, surgical resection by pancreaticoduodenectomy (PD) offers the only chance for cure (2-7).
Even after resection under optimal conditions, however, recurrence is the rule (2,8). The poor prognosis of these diseases has been thoroughly analyzed and several poor prognostic factors have been identified one of the most important observations is that periampullary carcinoma prognosis is closely related to the clinical stage (9). The categories of clinical stage (Table 1) defined by Fisher et al. and Warshaw et al. (10,11) are well known to surgeons familiar with PD. Surgical cure is rare in locally advanced lesions, hence the modifier “unresectable”. An opportunity for surgical cure does exist in lesions deemed to be resectable or borderline-resectable (11). The rationale for this clinical staging scheme hinges on the importance of the pathologic status of the surgical margins and the recognition of the impact of margin status on prognosis (6,7,9,10,12). Others have observed that patients left with a positive margin (R1 or R2) after PD for cancer of the HOP experienced a median survival that was similar to patients with localized (non-metastatic) disease who did not undergo resection, while those with grossly and microscopically negative (R0) margins after PD enjoyed an apparent survival advantage (3,13).
Full table
These data have led to the following principles: (I) when attempting to resect periampullary carcinoma, the goal should be to achieve an R0 resection (11); and (II) proper patient selection and operative technique are crucial to the effort of achieving negative margins (4-7,14-17).
Wolff et al. emphasized the importance of both high quality, multi-phasic CT examination in patient selection and the surgical clearance of the superior mesenteric artery (SMA) margin to achieving negative margins (18). These principles have represented a paradigm shift in the care of patients with periampullary carcinoma. It is unclear how widely these practices have been incorporated into direct patient management or what impact they have had on the success of margin clearance and overall survival (OS) in pancreatic cancer. We hypothesized that: (I) patients who had undergone operative clearance of the SMA margin by dissection along the right lateral wall of the SMA would be more likely to have had an R0 resection than patients who did not undergo this SMA dissection; and that (II) this improvement in R0 resection rates would result in an improvement in OS. In order to explore this hypothesis, we performed a single-institution, retrospective study of consecutive patients who underwent PD for periamplullary carcinoma to determine margin status and the impact of margin status on OS of patients in the cohort.
Methods
We performed a retrospective review of our in-house tumor registry to identify patients who underwent a PD for PA between January 1, 1985 and July 31, 2007. Eligibility criteria consisted of patients with a histologic diagnosis of adenocarcinoma of the HOP, ampulla, duodenum, or bile-duct, collectively called ampullary adenocarcinoma. A list of 509 eligible patients diagnosed with pancreatic cancer during the period of the study was initially identified. Patients who had not undergone a PD for their adenocarcinoma were eliminated, resulting in a final list of 93 patients for study. From a review of the medical records, we developed a custom database (Filemaker Pro, Filemaker, Inc.), which included pertinent demographic and clinical information.
In addition, pathology reports (Copath, Mysis, Inc.) were reviewed to determine the status of the common bile duct, pancreatic neck and SMA margins. Operative reports (Netaccess) were reviewed to determine which of the following operative maneuvers were performed: conventional PD, pylorus-sparing PD, or resection of SMV/PV. The impact of the various surgical maneuvers on margin status was examined using Fisher’s exact test.
We also analyzed the changes in operative techniques that occurred over time using the Chi-squared test for trend. The impact of margin status on OS was examined using the Kaplan-Meier method and log rank testing. P values less than or equal to 0.05 were considered statistically significant.
Results
A total of 93 patients were identified for inclusion in the study. For purposes of statistical analysis, the cohort was divided into early [1985-1998], middle [1999-2003] and late [2004-2007] groups.
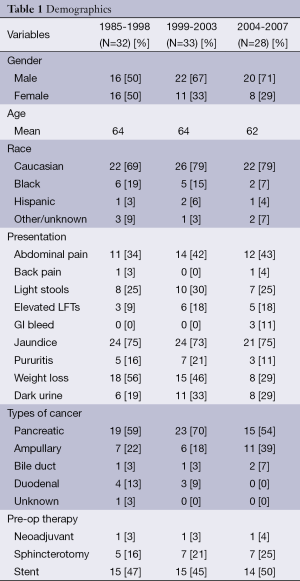
The study population between the groups was relatively stable in that gender, age, race, modes of presentation, types of cancer and types of preoperative therapy were not significantly different when compared among the three time periods (Table 1). Over the course of the study, with 98% (n=91) of the patients having nodal status evaluated, there was an increase in the relative proportion of lymph node (LN) positive tumors identified, with the positive nodal status increasing along with the number of LNs examined (Table 2). Over this same time period, the 30-day and in-house operative mortality decreased from 15.6% in the early group to 4% in the late group (19,20).
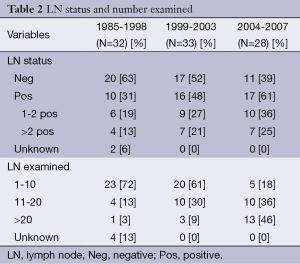
Full table
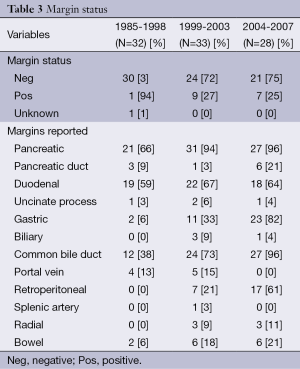
Overall, 18% (n=17) of cases were classified as having involved margins (Table 3). In the early group, only 3% (n=1) of cases had positive margins while in the latter two groups the margins were positive in about a quarter of cases [1999-2003, 27% (n=9); 2004-2007, 25% (n=7)]. However, no statistically significant association between margin status and any operative maneuver could be identified. Specifically, the likelihood of obtaining a negative margin was not increased among patients in whom the SMA dissection was described when compared to those patients in whom this dissection was not described.
Full table
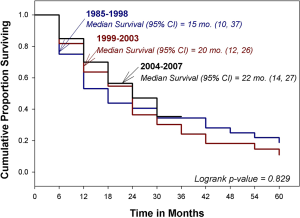
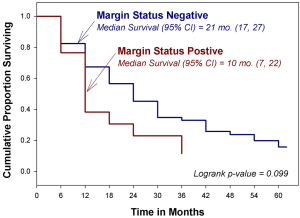
The median OS for the entire cohort was 19 months, with an estimated 5-year OS of 18% (Figure 1). Survival did not vary by time period, margin status, or the performance of an SMA dissection (Figure 2).
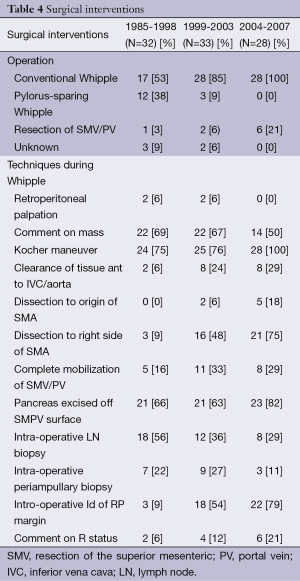
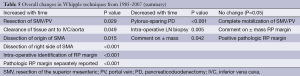
In contradiction to the apparent stability of our patient population, there were significant changes in the types of operations performed over the study time period. Pylorus-preserving resection declined during the study from 38% of cases early on to 0% in the late group, while resection of the superior mesenteric and/or the portal vein (SMV/PV) actually increased during the time period, accounting for about 1 in 5 cases in the late group (Table 4). In addition, intra-operative techniques such as intro-operative LN and periampullary biopsies decreased during this time period (56-29% and 22-11%, respectively), other techniques such as dissection of the SMA (both origin and right side) and intraoperative identification of the RP margin steadily increased (0-18%; 3-21%; 3-79%, respectively; Table 4). Dissection along the right side of the SMA was more common as time went on as was intra-operative identification and marking of the SMA margin. Finally, we observed that the three margins of interest—the common bile duct, pancreatic neck and SMA margins—were reported more frequently as time went on (Figure 1). The CBD margin was reported in only 38% of cases during the early time period but in 96% of cases by the end of the study period. Reporting of the pancreatic neck margin increased as well, from 66% to 96% of cases. The most dramatic change was seen in SMA margin reporting. In the early group, no pathology report made specific mention of this margin, but in the middle and late groups 21% and 61% of reports, respectively, made separate mention of the SMA margin. Overall, the summary of changes in the treatment of PD from 1995-2007 at our institution can be seen in Table 5.
Full table
Full table
Discussion
Although PD is a requisite part of the curative treatment for periampullary carcinoma (9), the low cure rates after surgery alone (3,9,21) and the potential morbidity and mortality of the procedure (1,22) make proper patient selection and conduct of the operation important aspects in the care these patients. Since clearance of the surgical margins is associated with a survival advantage (9,10,23), both the patient selection process and operative techniques should be aimed at optimizing the likelihood of achieving an R0 margin status. In spite of general agreement on the importance of proper patient selection and operative techniques (16,17,24), few studies have attempted to describe the extent to which the selection process and crucial operative techniques have been incorporated into clinical practice or correlate these techniques with margin status.
In the current study, we were unable to demonstrate that focused surgical attention to the SMA margin was associated with improvements in OS or with higher R0 resection rates. This lack of an association between SMA margin and OS likely stems from multiple factors; first, close attention to the SMA margin appeared to be a rather late-developing phenomenon. It is possible that the low rate of positive margins observed during the early time period represents an underestimation of the true rate. Such an underestimation is plausible in light of our observation that the key margins were reported in a minority of early patients. Considering this differential in margin reporting, our observation of higher rates of margin positive resections in the middle and late groups is not surprising. One could argue that the increased attention paid to the margins by the surgeons resulted in a closer assessment of the margins by the pathologists. And, in fact, the increased marking of the SMA margin by the surgeon coincided with the increased identification of it by the pathologist, thus supporting this argument. Certainly, it has been recognized that retrospective assessment of margins is difficult and that real time orientation of the specimen and identification of the margins is required for an accurate assessment of the margins. In any case, the potential misclassification of margin status in the early group might have masked any advantage provided by dissection along the SMA that took place in the middle and late groups.
Another potential explanation for the lack of association between SMA dissection and OS/R0 resection rates is a shift in the complexity of the patients over the course of the study. The increases in node positive disease and portal vein resections that we observed in the middle and late periods suggest that these patients were likely a higher risk group with more aggressive tumor biology. These indicators of aggressive biology would be expected to be associated with a higher risk of microscopically positive margin involvement (25). In addition, the average number of cases per year in each cohort, which increased from about two per year in the early group to eight per year in the late, also suggests that the patients in the early group might have been a more highly selected patient population and could have represented the lowest risk patients presenting during that time period (19,20). If such differences in disease biology did exist, the patients in the early group would be expected to do better than those in the middle and late groups. Therefore, any advantage derived from SMA dissection in the middle and late groups might not have been evident.
Another potential shortcoming of our analysis is the sole focus on operative technique. Certainly, while we believe that operative technique is an important determinant of margin status, we also recognize that it is not the only factor that has an impact. A more sophisticated, multivariate analysis of the factors that are associated with margin status was not possible due to the size of our study. And, in fact, the modest size of our cohort suggests the potential for a type 2 error due to inadequate power.
An alternate explanation for the lack of association between operative technique and margin status is that the operative reports might not accurately represent what was actually done. Since this was a retrospective study, we were forced to rely on the operative reports to define the surgical maneuvers employed. It is possible that similar operative techniques were performed by surgeons throughout the study period but merely reported differently over time. The convergence of the operative data and the pathologic data, however, make that unlikely.
Finally, we have to acknowledge that the lack of an association between margin status and technique and margin status and survival could be because no such association exists. Although our data cannot refute this possibility directly, the weight of previous studies and the opinion of pancreatic cancer experts both support a link between these factors (2,9,25). In spite of the fact that our study failed to add to that evidence, it has given us insight into the past and current status of pancreatic surgery and the pathologic assessment of PD specimens at our institution.
We now understand that there have been changes in the conduct of PD over the years within our study [1987-2007]. Pylorus-preserving PD is on the decline while portal vein resections have become more common. The importance of this change in operative technique is not clear, but it seems unlikely that the switch away from pylorus preservation would have a cause-and-effect relationship with the higher rates of positive margins we observed throughout the study. A more plausible explanation for the increase in margin positivity is the combination of more aggressive disease biology and a more thorough, and therefore accurate, assessment of the surgical margins. Our finding that reporting of surgical margins improved over time and that, specifically, attention to the SMA margin has grown (5,10,25), supports this theory.
In spite of the fact that the current study failed to confirm our main hypothesis, we did gain valuable insight about the management of PD and the pathologic assessment these specimens at our institution.
Future quality improvement efforts planned at our institution will implement standardized operative templates that would require a surgeon to indicate, in the affirmative or negative, whether a particular part of the procedure was performed. In addition, intra-operative interaction between surgeon and pathologist to orient the specimen and identify the crucial margins of interest will be strongly encouraged. Prospective recording and reporting of these activities could serve as surrogate indicators for quality of care, not unlike the requirement for surgeons participating in ACOSOG-Z5041 to obtain intra-operative photographs of the SMA and SMV/PV to document appropriate clearance of these margins. These surrogate quality indicators might be an especially useful adjunct in evaluating pancreatic surgery programs with more moderate volumes, since estimates of survival and operative complications in such programs are susceptible to wide variation after only a few adverse events and, thus, can be somewhat imprecise, with wide confidence intervals. Finally, introduction of synoptic pathology reports that require separate reporting of the margins is now required by the most recent iteration of the CAP guidelines (26).
Prospective recording of operative details, real-time, in-person communication between surgeon and pathologist, and standardization of the pathology report to include critical information should increase our ability to verify whether proper surgical techniques are employed on a consistent basis and, ultimately, to correlate the use of these techniques with important outcomes. We believe these changes are necessary to provide optimal care to patients with PA. By implementing them, pancreatic surgery programs will signal their interest in this process to those involved in efforts to develop strategies for the regionalization of care of such complex clinical problems.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248-57; discussion 257-60. [PubMed]
- Yeo CJ. Periampullary cancer. In: Cameron JL, editor. Current Surgical Therapy. 6th ed. Mosby: St. Louis, 1998:520-27.
- Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996;223:273-9. [PubMed]
- Andersen DK, Brunicardi C. Essentials of surgery: scientific principles and practice. In: Greenfield LJ, Mulholland MW, Oldham KT, et al, editors. Pancreatic Anatomy and Physiology. Lippincott: Williams & Wilkins, 1997:235-41.
- Bell RH. Neoplasms of the Exocrine Pancreas. In: Greenfield LJ, Mulholland MW, Oldham KT, editors. Essentials of Surgery: Scientific Principles and Practice. Philadelphia: Lippincott-Raven, 1997:253-58.
- Fernandez-del-Castillo C, Jimenez RE, Steer ML. Surgery in the Treatment of Exocrine Pancreas and Prognosis. Available online: http://deu.uptodate.com/physicians/oncology_toclist.asp
- Steer ML. Exocrine Pancreas. In: Townsend CM, Beauchamp DR, Evers MB, et al, editors. Sabiston Textbook of Surgery The Biological Basis of Modern Surgical Practice. Philadelphia: Saunders, 2004:1643-78.
- Yen TW, Abdalla EK, Pisters PW, et al. Pancreaticoduodenectomy. In: VonHoff DD, Evans DB, Hruban RH, editors. Pancreatic Cancer. Sudbury: Jones & Bartlett, 2005:265-83.
- Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995;221:59-66. [PubMed]
- Fisher WE, Andersen DK, Saluja AK. Schwartz’s Principles of Surgery. In: Charles Brunicardi F, Andersen DK, Billiar TR, et al, editors. Pancreas. New York: McGraw-Hill, 2005:1221-96.
- Warshaw AL, Gu ZY, Wittenberg J, et al. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg 1990;125:230-3. [PubMed]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721-31; discussion 731-3. [PubMed]
- Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001;8:123-32. [PubMed]
- Verbeke CS. Resection margins in pancreatic cancer. Surg Clin North Am 2013;93:647-62. [PubMed]
- Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993;217:430-5; discussion 435-8. [PubMed]
- Lerut JP, Gianello PR, Otte JB, et al. Pancreaticoduodenal resection. Surgical experience and evaluation of risk factors in 103 patients. Ann Surg 1984;199:432-7. [PubMed]
- Lillemoe KD, Sauter PK, Pitt HA, et al. Current status of surgical palliation of periampullary carcinoma. Surg Gynecol Obstet 1993;176:1-10. [PubMed]
- Wolff RA, Abbruzzese J, Evans DB. Treatment of localized, potentially resectable disease. In: Kufe DW, Pollock RE, Weichselbaum RR, et al, editors. Hamilton, ON: BC Decker, 2003.
- Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 1999;125:250-6. [PubMed]
- Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 1999;126:178-83. [PubMed]
- Metreveli RE, Sahm K, Abdel-Misih R, et al. Major pancreatic resections for suspected cancer in a community-based teaching hospital: lessons learned. J Surg Oncol 2007;95:201-6. [PubMed]
- Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer 1990;66:56-61. [PubMed]
- Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg 1987;206:358-65. [PubMed]
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447-58. [PubMed]
- Winter JM, Cameron JL, Yeo CJ, et al. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg 2007;204:1029-36; discussion 1037-8. [PubMed]
- Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. Available online: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/PancreasExo_12protocol_3200.pdf