Thrombotic events in metastatic colorectal cancer patients treated with leucovorin, fluorouracil and irinotican (FOLFIRI) plus bevacizumab
Background
Bevacizumab (BV, Avastin®) is a recombinant humanized monoclonal antibody directed against vascular endothelial growth factor (VEGF), an important regulator of angiogenesis (1). Given its crucial role in inhibiting angiogenesis, this biologic agent has been utilized in the treatment of colon (2-4), ovarian (5), and breast cancers (6), as well as brain tumors (glioblastoma multiforme) (7). The most established use of BV is in the first and second line treatment of metastatic colorectal cancer (mCRC). A survival benefit in mCRC cancer patients who received BV combined with standard 5-FU-based chemotherapy regimens has been demonstrated in several phase II and III studies (2-4). However, its impact on overall survival is relatively modest and its use is associated with several serious, albeit relatively rare side effects (8-10). The principally reported adverse effects of BV are arterial hypertension, arterial and venous thrombosis, cardiovascular events, bowel perforation, bleeding, and proteinuria (8-10).
It has been shown that BV is associated with increased treatment-related fatal adverse effects with an RR of 1.33 (95% CI, 1.02-1.73; P=0.04; incidence, 2.9% vs. 2.2%) when used in combination with chemotherapy or biological therapy, compared to chemotherapy alone (7). The likelihood of these adverse events is thought to be increased in patients with certain pre-existing conditions, including thrombosis, hemorrhage, or uncontrolled hypertension. In another meta-analysis, the summary incidence of all-grade and high-grade venous thromboembolic events (VTE) were 11.9% (95%CI: 6.8-19.9%) and 6.3% (95% CI: 4.8-8.3%), respectively (11).
The link between cancer and thrombosis is well established (8), and the prothrombotic state induced by malignancy can be exacerbated further by chemotherapy, hormone therapy, biological therapy, and surgery (8). The exact mechanism of BV induced thrombosis is poorly understood, however, BV has been found to induce platelet aggregation, degranulation, and thrombosis during complex formation with VEGF, and to induce activation of the platelet FcγRIIa receptor (12).
This single-center retrospective cohort study, conducted at the Juravinski Cancer Centre, a large academic cancer center in Ontario, Canada, was designed to determine whether BV use in patients with mCRC is associated with an increased risk of thrombosis compared to chemotherapy alone.
Methods
Data source
Ethics approval for this project was obtained from the local Research Ethics Board. Study data were sourced from a registry of patients diagnosed with mCRC at Juravinski Cancer Centre in Hamilton, Ontario, Canada. This database is a comprehensive electronic medical charting system that includes all patients with a pathologically confirmed cancer diagnosis, referred to the center from facilities within the center’s catchment area, in addition to those referred from other cancer centers. Chemotherapy protocols, doses and administration for each patient were cross-checked utilizing the cancer center pharmacy records. No discrepancies were observed between the two sources of the study data.
Patients
The control group consisted of patients with metastatic colorectal carcinoma who were treated, with FOLFIRI alone between April 2004 and September 2011. The reasons patients in the control group were not given BV in addition to FOLFIRI are summarized in Table 1. In September 2006, BV became a publicly funded drug to be used in combination with FOLFIRI for treatment of mCRC. The use of chemotherapy in Ontario is guided by Cancer Care Ontario, which is an evidence based group of experts who prepare guidelines and approve chemotherapy regimens and other anticancer agents based on available evidence and cost effectiveness of the agents. The criteria for the use of chemotherapy regimens in Ontario are stringent and evidence informed resulting in a relatively homogeneous group of patients receiving the same treatments during a particular time period for a specific malignancy.

Full table
The experimental group consisted of patients treated with FOLFIRI plus BV between August 2006 and November 2011. Patients who received BV alone as a single agent or with chemotherapeutic agents other than FOLFIRI were excluded.
Data collection
Information on patient demographics (sex and age) and clinical characteristics (BMI, medical conditions/co-morbidities, primary tumor location (colon, rectosigmoid, rectal), sites of metastases, and risk factors for venous thromboembolism (VTE) or arterial thrombosis) were obtained. VTE risk factors of interest included past history of deep vein thrombosis (DVT) or pulmonary embolus (PE), prior superficial thrombophlebitis, surgery requiring general anesthesia for more than 30 min, the use of Erythropoietin (Eprex), or any other antineoplastic agents within 4 weeks of TEs. Risk factors for arterial thrombosis included past history of myocardial infarction, angina, stroke, diabetes, hypertension, hyperlipidemia, atrial fibrillation, smoking, or a family history of myocardial infarction, stroke, or transient ischemic attacks (TIA). The above information was collected for both groups independently by two different investigators (HOA and MA). The data were verified by a third investigator. Any data conflicts were resolved by consensus (HOA, MA, and AA).
Outcome measures
Thrombotic events (TEs) were documented by reviewing electronic medical records and imaging reports. A diagnosis of venous thrombosis was based on the description by the original reporting radiologist. The cytotoxic pharmacy records were utilized to verify when treatment was stopped or when chemotherapeutic agents were omitted from a regimen. However, the reasons for discontinuation of the BV were not provided in the pharmacy records. The dates and number of cycles of antineoplastic treatment prior to diagnosis with a TE were recorded. Site of VTE (lower limb, upper limb, pulmonary, others) and setting of diagnosis (incidental, symptomatic) were identified for both study groups. The dates to first TE in relation to the start of FOLFIRI chemotherapy protocol (+/- BV) were also collected.
Statistical analysis
Descriptive statistics were used to summarize the study population demographics and clinical characteristics using R (V2.15.3). Student’s t-tests (continuous variables) and chi-square tests (categorical variables) were used to perform comparisons of patient demographic, clinical, and treatment characteristics, as well as clinical outcomes. Regression analyses were carried out to adjust for the effect of confounding variables between the addition of BV and the incidence of TEs. A P value of <0.05 was considered statistically significant.
Results
Patients
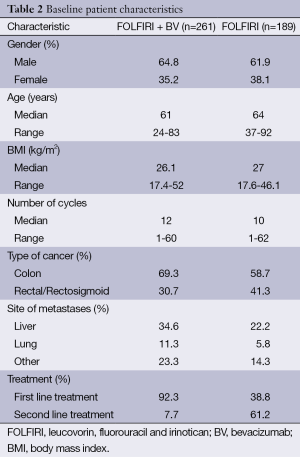
A total of 450 patients were identified: 261 in the FOLFIRI plus BV group, and 189 in the FOLFIRI alone group. Patient demographics are shown in Table 2. In the FOLFIRI plus BV group, the median age was 61 years and 64.8% were male. In the FOLFIRI alone group, the median age was 64 years and 62% were male. There was no statistically significant difference in median BMI or in the number of cycles between the two groups. The proportion of patients who had colon cancer was higher in the FOLFIRI plus BV group (69.3% vs. 58.7%), whereas the proportion of patients who had rectal cancer was higher in the FOLFIRI alone group (41.3% vs. 30.7%). Of the patients in the FOLFIRI plus BV group, 92.3% received this treatment as a first line therapy, whereas 61.2% received FOLFIRI alone as first line treatment.
Full table
Thrombotic events (TEs)
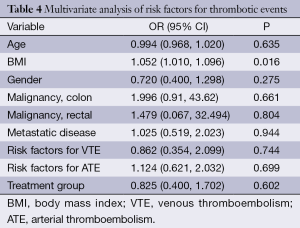
The incidence of TE was 14.9% (39/261) in the FOLFIRI plus BV group compared to 15.9% (30/189) in the FOLFIRI alone group (P=0.24). There was no statistically significant difference in the rates of TE based on thrombus location. There were no documented deaths reported due to TE in either group. The details of the TEs are presented in Table 3. The median time-to-event for TE in the FOLFIRI plus BV group was earlier compared with the FOLFIRI alone group (P=0.002). The results of multivariate regression analysis controlling for age, gender, malignancy, metastatic disease, and line of treatment are shown in Table 4. These results do not suggest a significant increase in the risk of thrombosis with FOLFIRI plus BV (OR =0.83; 95% CI: 0.40-1.70; P=0.602).

Full table
Full table
Risk factors
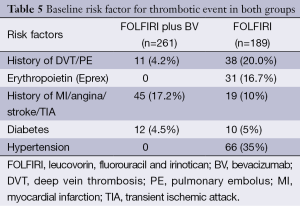
Baseline risk factors for TEs in both groups were recorded (Table 5). A higher number of patients in the FOLFIRI alone group had prior DVT/PE compared with the FOLFIRI alone group (20% and 4.2%, respectively). In addition, patients in the FOLFIRI alone group were more likely to have a history of MI/angina/stroke/TIA (17.2%) and hypertension (35%).
Full table
The results of the multivariate logistic regression analysis of baseline risk factors for TE are shown in Table 4. In the BV group, there was no significant association between any of the risk factors and thrombosis (OR =0.83; 95% CI: 0.40-1.70; P=0.602). The only risk factor associated with a statistically higher risk of thrombosis in the FOLFIRI plus BV group was increased BMI (OR =1.05; 95% CI: 1.01-1.10; P=0.02). There was no significant difference according to treatment group (OR =0.83; 95% CI: 0.40-1.70; P=0.60).
Discussion
The findings of this retrospective analysis suggest that the addition of BV to FOLIRI alone in patients with mCRC is not associated with an increased risk of thrombosis. Among the various risk factors for TEs, only increased BMI was associated with a significant increase in the risk of thrombosis in the BV plus FOLFIRI group (OR =1.05; 95% CI: 1.01-1.10; P=0.01).
This study, to our knowledge, is the first one to evaluate the incidence of TE in patients with mCRC who are receiving BV plus FOLFIRI. The incidence of VTE in association with BV was examined in a meta-analysis by Meyer et al., which reported rates of all-grade and high-grade VTE of 11.9% (95% CI: 6.8-19.9%) and 6.3% (95% CI: 4.8-8.3%), respectively (11). This meta-analysis included patients who received BV for different indications and with different chemotherapy combinations. The only other study that assessed the efficacy of FOLFIRI in combination with BV (compared with FOLFIRI alone) in patients with mCRC did not report the thrombotic rate (13).
In our study, the incidence of thrombosis in the FOLFIRI plus BV group was not statistically different from the FOLFIRI alone group (14.9% vs. 15.9% respectively, P=0.24). Furthermore, there was no statistically significant difference in the site of the TE between the two groups. Interestingly, no arterial TEs were reported in either group. Our reported TE rates were similar to those reported by Hurwitz et al. (2) in their Phase III study, where the efficacy of BV in combination with fluorouracil and leucovorin (IFL) versus IFL alone were assessed in a first-line setting in mCRC. The incidence of all venous and arterial TEs was 19.4% in the group given IFL plus BV and 16.2% in the group given IFL plus placebo (P=0.26) (2). A breakdown of the TEs was not provided in this study. There were no documented fatalities reported in the current study attributed to TEs, however, this cannot be confirmed because of the retrospective nature of the study.
The imbalance in the baseline risk factor for TE in both groups is important to note. The FOLFIRI alone group had a higher rate of prior DVT/PE (20%) compared with in the FOLFIRI plus BV group (4.2%). This discrepancy is likely due to concern about an increased risk of thrombosis with the addition of BV. The time to TE was significantly shorter in the FOLFIRI plus BV group (96 days) as compared with 141 days in the FOLFIRI alone group (P=0.03). This suggests that the addition of BV may initially induce a higher prothrombotic state compared to FOLFIRI alone, however the total number of TEs is similar to that seen with FOLFIRI alone.
Multivariate analyses were used to identify factors that might increase the risk of TEs. In the FOLFIRI plus BV group, no significant association was observed between the studied risk factors and thrombosis (OR =0.83; 95% CI: 0.40-1.70; P=0.602). Increased BMI was the only significant risk factor found to be associated with thrombosis in the BV group (OR =1.05; 95% CI: 1.01-1.10; P=0.01). Obesity, defined by BMI of ≥30 kg/m2 is known to be associated with an increased risk for thrombosis, independent of malignancy (14-16). Since the BV dose is calculated based on weight, a potential explanation for this finding is the higher total dose of BV given to patients with higher BMI.
Our results contrast with those of the meta-analysis by Nalluri et al. (8) which found an increased risk of VTE associated with BV in patients with various malignancies. However, our study analyzed a more homogeneous patient population (mCRC patients given the same regimen). Our study is limited by the retrospective nature of the analysis with the potential for under-reporting of TEs. The imbalance in TE risk factors between the groups is also an important limitation which suggests that our conclusions cannot be applied to patients with a past history of VTE who are treated with FOLFIRI plus BV.
Conclusions
Our findings suggest that the addition of BV to FOLFIRI for treatment of patients with mCRC does not significantly increase the rate of thrombosis in patients without a history of VTE compared with FOLFIRI alone. Increased BMI may be a risk factor for thrombosis in patients treated with BV, but this requires further evaluation in future studies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 2005;23:3502-8. [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Saif MW, Mehra R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf 2006;5:553-66. [PubMed]
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 2008;26:3523-9. [PubMed]
- Tebbutt NC, Murphy F, Zannino D, et al. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol 2011;22:1834-8. [PubMed]
- Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA 2011;305:487-94. [PubMed]
- Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008;300:2277-85. [PubMed]
- Evidence-Based guidelines. Available online: https://www.cancercare.on.ca/toolbox/qualityguidelines/, accessed Jan 14th 2015.
- Stathopoulos GP, Batziou C, Trafalis D, et al. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology 2010;78:376-81. [PubMed]
- Meyer T, Robles-Carrillo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost 2009;7:171-81. [PubMed]
- Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484-96. [PubMed]
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [PubMed]
- Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93-102. [PubMed]
- Romualdi E, Squizzato A, Ageno W. Abdominal obesity and the risk of recurrent deep vein thrombosis. Thromb Res 2007;119:687-90. [PubMed]
- Eichinger S, Hron G, Bialonczyk C, et al. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med 2008;168:1678-83. [PubMed]


