Regression of hepatocellular carcinoma after treatment of hepatitis C: a case report
Introduction
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver that could be caused by chronic hepatitis C induced liver cirrhosis (1). Treatment strategies for HCC involve surgical resection, liver transplant or locoregional therapy such as radiofrequency ablation (RFA) and transarterial chemoembolization (TACE) (2,3). Patients who are not candidates for surgery or locoregional therapy are considered for systemic therapy with sorafenib (4). Regimens to treat hepatitis C infection include interferon based therapy or a combination of ribavirin and other new generation anti-viral agents such as sofosbuvir (5,6). Whether these new anti-hepaciviral regimens could have an impact on HCC remains to be determined. We report a case of metastatic HCC who failed treatment with sorafenib, but tumor regressed after the patient was subsequently treated for hepatitis C with ribavirin and sofosbuvir.
The case
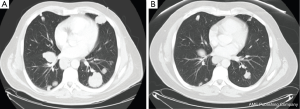
A 53-year-old male with a history of hepatitis C (genotype 1b) diagnosed in 2002 and HCC that was diagnosed in January 2013. The patient received 12 weeks of pegylated interferon and ribavirin for Hepatitis C in 2003 that was unsuccessful. The patient was found to have an elevated AFP of 79 in August 2012 and liver cirrhosis. In January 2013, he was found to have a 3.2 cm liver lesion that was biopsied and pathology report was consistent with HCC. Accordingly, the patient underwent RFA of the liver lesion on January 2013 followed by TACE for another 7 mm lesion. Since the tumor was within the Milan Criteria, the patient had a liver transplant at University of Virginia (UVA) in August 2013. Subsequently, he developed lung metastases that were confirmed by biopsy on December 2013. Therefore, sorafenib was started on December 2013 but unfortunately the patient developed progression of disease in March 2014 described as an enlargement in the existing lung lesions in addition to new lung lesions. Accordingly, sorafenib was stopped. In March 2014, the patient developed an elevation of his total bilirubin to 11, elevated liver function tests, and ascites that was thought to be related to HCC and HCV infection (viral load was 26.5 million IU/mL). The patient was successfully treated for his HCV with ribavirin and sofosbuvir for 24 weeks and completed treatment in the end of August 2014. Repeat hepatitis C viral load after 6 weeks of treatment was less than 43 IU/mL. While receiving treatment for Hepatitis C, the patient had further evidence of HCC progression by CT scans in June 2014. He was then referred to Virginia Commonwealth University as a potential candidate for a clinical trial; however, a restaging chest CT scan was performed on 9/5/14 and showed significant reduction in the size of pulmonary metastatic lesions compared to CT scan in June 2014 with the largest mass in the right lower lobe measuring 2.0 cm × 2.3 cm compared to 3.6 cm × 4.3 cm previously (Figure 1). Of note, post-transplant, the patient was placed on immunosuppression with tacrolimus and low dose everolimus that was continued during his treatment course.
Discussion
This patient failed treatment with sorafenib as evident by progressive disease on CT scans after 3 months of therapy then subsequently received treatment for hepatitis C with ribavirin and sofosbuvir for 24 weeks and repeated imaging showed regression of lung metastases. Sofosbuvir is an anti-hepaciviral polymerase inhibitor that is used in combination with ribavirin to treat hepatitis C infection (6,7). Sofosbuvir, a direct-acting antiviral agent against the hepatitis C virus, is a prodrug converted to its pharmacologically active form (GS-461203) via intracellular metabolism. It inhibits HCV NS5B RNA-dependent RNA polymerase that is essential for viral replication and acts as a chain terminator (8). Ribavirin is an anti-hepaciviral nucleoside analogue which inhibits replication of RNA and DNA viruses and inhibits the initiation and elongation of RNA fragments resulting in inhibition of viral protein synthesis (9). The combination of sofosbuvir and ribavirin is currently used in hepatitis C patients waiting for liver transplant to treat HCV infection prior to transplant with the goal of achieving sustained viral response and prevent infection of the transplanted liver (10). This combination was studied in patients with HCC awaiting liver transplant, however, there was no mention as to whether any of these patients had regression of their tumor before transplant (10). Ribavirin inhibits p38 mitogen-activated protein kinases (MAPK) phosphorylation in cells infected with HCV resulting in anti-viral affect (11). Knockdown of this pathway may have contributed to the anti-tumor effect in this patient (12). The patient was also on low dose everolimus (1 mg daily) for immunosuppression. Everolimus is an mTOR inhibitor but its anti-tumor effect is observed at much higher doses (7.5-daily) (13). Everolimus did not appear to have significant improvement in overall survival in patients with HCC who had failed sorafenib or intolerant to sorafenib although there was a significant improvement in disease control rate compared to placebo (12). Low dose everolimus is unlikely to have contributed to the regression of this patient’s tumor since this patient had been on everolimus since his liver transplant and had disease progression while on it. To our knowledge, this is the first reported case of HCC tumor regression after treatment of hepatitis C. More studies are needed to explore the impact of hepatitis C treatment with anti-hepaciviral regimens on HCC in patients with hepatitis C induced liver cirrhosis and HCC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529-38. [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Chevaliez S, Pawlotsky JM. Hepatitis C virus: virology, diagnosis and management of antiviral therapy. World J Gastroenterol 2007;13:2461-6. [PubMed]
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878-87. [PubMed]
- Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867-77. [PubMed]
- Cholongitas E, Papatheodoridis GV. Sofosbuvir: a novel oral agent for chronic hepatitis C. Ann Gastroenterol 2014;27:331-7. [PubMed]
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005;436:967-72. [PubMed]
- Curry MP, Forns X, Chung RT, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology 2015;148:100-7. [PubMed]
- He SF, Wang W, Ren H, et al. Interferon alpha and ribavirin collaboratively regulate p38 mitogen-activated protein kinase signaling in hepatoma cells. Cytokine 2013;61:801-7. [PubMed]
- Koul HK, Pal M, Koul S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer 2013;4:342-59. [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [PubMed]


