Duodenojejunal flexure tumors: surgical difficulties with case series
Introduction
The incidence of tumors in the duodenojejunal flexure is less in the entire gastrointestinal system. They attain importance because of the vague symptoms at presentation, rarity of location and the difficulty during attempts at resection. The incidence of duodenal tumors is only 33% to 45% of all the small bowel tumors and only 0.3% of all the digestive tract tumors (1,2). This low incidence of the duodenal tumors is attributed to the liquid component of the food material that decreases the transit time, thus decreasing the exposure, and a low bacterial count in the small bowel does not effectively convert the bile acids into potential carcinogens (3). Two thirds of duodenal tumors arise from the second part, around the ampullary region (4). The difficulty in operating these tumors due to their strategic location has led us to share our experience in operating up on these tumors.
Methods
A search was made through all the medical records of patients who were admitted and diagnosed to have duodenal tumors between January, 2011 and March, 2014 in Apollo Hospitals, Chennai, India. Search was made through our hospital database, using international code for diseases (ICD) classification. Patients with duodenal tumors arising from third or fourth part were selected for analysis. Nine patients were finally identified to have been treated for tumors in the distal duodenum and duodenojejunal flexure. Diagnosis was made by either endoscopy or radiological imaging on clinical suspicion and location confirmed intraoperatively, followed by histopathological diagnosis in all the patients.
Results
Nine patients, seven males and two females (male/female ratio, 3.5:1), were diagnosed to have tumor in the third and fourth part of the duodenum. The mean age at presentation is 48.67 years (youngest 28 and oldest 75 years of age). Three patients had tumor in the third part and six had tumor in the duodenojejunal flexure. Vomiting is the most common symptom in 55% cases followed by abdominal pain and weight loss in 33% patients. Abdominal distention, palpable epigastric mass, melena and pallor (22%, 2 patients each) are the most common signs. Palpable epigastric mass was associated with inoperability due to distant metastasis in one patient, and the mass was found to be a grossly dilated duodenum in the other with a stricturous lesion in the D4-Jenunal junction. Jaundice was seen in one patient due to enlarged nodes in hepatoduodenal ligament. Other symptoms include anorexia (2/9), melena (1/9) and dyspepsia (2/9 patients) (Table 1).
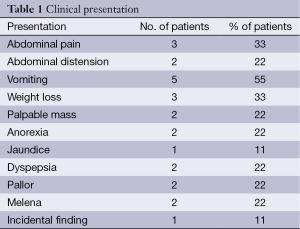
Full table
Endoscopy was successful in only two patients in identifying the location of the tumor. Contrast enhanced computed tomography (CECT) was successful in identifying the location of the tumor in six patients out eight in whom CECT was available (Figure 1), but failed to assess vascular involvement in one out of eight patients. It was successfully assessed distant metastasis in one patient. Pancreas is infiltrated focally in one of the nine patients. Barium study was not routine and was done only in one patient successfully localizing the tumor. Similar is the case with ultrasound abdomen, done in four patients identifying the lesion in one and failing in the other due to bowel gas (Table 2).
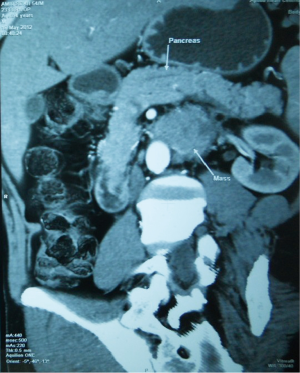
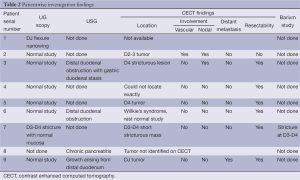
Full table
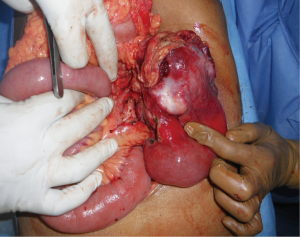
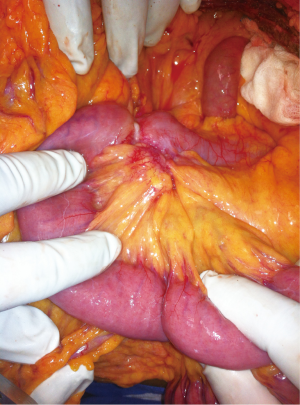
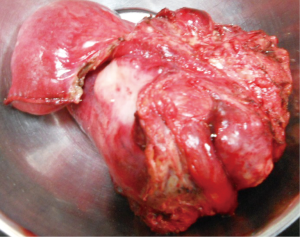
After investigating, all the patients underwent laparotomy with an intention to cure (Figure 2) except for one patient in whom metastasis was identified preoperatively and taken up for laparotomy for palliative surgery. At laparotomy, major vascular involvement was noticed in three out of nine patients (33%). It was superior mesenteric artery (SMA) involvement in three, superior mesenteric vein (SMV) involvement in one, inferior mesenteric vein (IMV) in one and jejunal branches involvement in one (Figure S1) of the nine patients [one had both SMA and jejunal vessel involvement, the other had SMA, SMV and IMV involvement]. Among the patients that underwent surgery with a curative intent, segmental resection was feasible in five out of six patients (Figure 3) (four underwent duodenojejunostomy and one underwent Roux-en-Y loop pancreaticojejunostomy, the resection of tumor segment followed by jejunojenunostomy). R0 resection was feasible only in five (55%) patients (one patient had abdominal wall metastasis and was excised with negative margins). R2 resection was done in one patient (tumor abutting SMA is shaved off from the surface of the vessel) as the patient had severe anemia due to chronic bleeding from the tumor. Three out of nine patients had a palliative surgery (33%, one due to distant metastasis and other two due to vascular involvement). One patient with focal infiltration into the pancreas head had undergone Whipple’s pancreaticoduodenectomy to achieve R0 resection (Table 3).
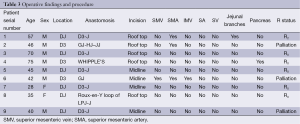
Full table
The most common histopathological type is adenocarcinoma in 66% patients (five moderately differentiated and one poorly differentiated). Both the patients with pallor and melena at presentation had Gastro intestinal stromal tumor (GIST). Pancreatic choriostoma was an incidental on operative table finding in a patient undergoing Frey’s lateral pancreaticojejunostomy for chronic pancreatitis. Of the five patients that had R0 margins at histopathology, four had moderately differentiated adenocarcinoma and one had GIST. Two patients with adenocarcinoma and the other metastatic GIST on biopsy had undergone palliative surgery. On final histopathology of the five resected patients, three had perineural invasion, one had vascular invasion and four had transmural spread on final histopathology. Totally 8 to 18 lymphnodes could be retrieved depending on the distal margin of the specimen. As the distal margin increased, the number of nodes retrieved increased (Table 4).
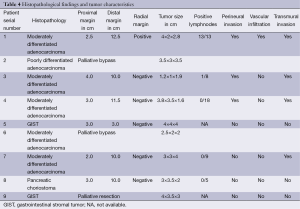
Full table
Discussion
The incidence of adenocarcinomas increase as we go from the first part to the fourth part of the duodenum (15% in first, 40% in second and 45% in 3rd and 4th parts together) and is attributed to the length of the duodenum that is exposed to the toxins during the transit (5,6). However, vague symptomatology result in delayed presentation and pose difficulties not only in diagnosing, but also in offering curative treatment to these patients. And also, the strategic location of the DJ flexure tumors close to the major vessels like SMA and vein, transverse mesocolic vessels, proximal jejunal vessels, IMV, splenic vein, proximity to important viscera like pancreas pose a challenge to the surgeon to offer R0 resection.
Though biopsy is accepted as gold standard for diagnosing gastrointestinal tumors, the modality in performing one is hard to choose mainly due to the difficulty in reaching these tumors through a standard esophagogastroduodenoscopy and due to major vessels in the surrounding to approach under imaging guidance. However, in our experience most of the patients presented with symptomatology mimicking proximal small bowel obstruction. This has made us deviate from the standard preoperative biopsy notion to an intra-operative biopsy (as a part of palliative procedure) or excisional biopsy (in an attempt to achieve R0 margins) as the case may be depending on the resectability—partly based on the imaging and partly on the intraoperative findings. The limited data in the literature does not suggest or support any radicality in lymph nodal resections or the margins. However, as they seem to behave the same way as any tumor in rest of the intestine in accordance with the histological type, we believe the same principles can be applied in case of DJ flexure tumors also. In fact a study form Lahey Clinic Medical center has shown survival of patients with adenocarcinoma of the distal duodenum is good when compared to the proximal duodenal tumors, and segmental resection is the procedure of choice (7). Hence, every attempt should be made for an R0 clearance with less morbid procedure like segmental resection.
In our case series, symptoms are insidious at the onset with mean time to onset and presentation of about 5 months. Symptomatology is suggestive of proximal small bowel obstructions with vomiting being the most common clinical presentation in our series. However, earlier reported case series on DJ flexure tumors have noticed vague abdominal pain as the most common symptom (8). Palpable epigastric mass seems to be a sign of advanced disease suggesting inoperability. CECT is the investigation of choice among the surgeons as it gives useful information regarding the location, vascular involvement, local organ infiltration and distant metastasis when the EGD scope fails to locate the site of obstruction. R0 resection is possible in about 50% of the patients and most can be achieved by a simple segmental resection rather than a complex surgery like a Whipple’s pancreaticoduodenctomy. Adenocarcinoma is the most common histopathological variety in our series. There was no perioperative mortality and only one patient had chylous ascites which was managed conservatively. There were no pancreatic fistulas or anastomotic leaks in the series. On final histopathology, a proximal negative margin of minimum 2 cm could be achieved in all the resectable tumors. Venous resections were not required in any of the patients. The number of positive lymph nodes on final histopathology increased proportionately to the vascular invasion on microscopy, and it expressed itself as a locally advanced tumor.
In none of the cases, vascular resections and grafting was done in our series. But, they did pose some problems in clearing the tumors off the superior mesenteric vessels, splenic vein, pancreatic inferior margin and while traversing through the root of the mesentery. Though we did not encounter, when infiltrated, splenic vessels may be sacrificed by performing a splenectomy, may be as a part of distal pancreatectomy. When the inferior margin of the pancreas is involved a Whipple’s pancreaticoduodenectomy (in one patient of our series) or distal pancreatectomy can be performed in a standard way if it can result in a R0 resection. But shaving off the tumor from pancreatic surface in this situation is not advised as it can cause pancreatic fistula and thus can add to the morbidity of the patient. Involvement of the superior mesenteric vessels does put a surgeon in dilemma, as there is no literature support to vascular resection and grafting. Any attempt at it is indeed experimental. We did come across a situation when the tumor infiltrated on to the SMA, SMV and IMV, and we have resorted to palliative procedure. However an isolated IMV involvement can be safely dealt with ligation and excision without reconstruction in order to achieve a R0 resection. We noticed that the posterior margins did become important in these DJ tumors as it becomes intra-peritoneal from retroperitoneal position. After segmental resection, anastomosis between third part of duodenum (D3) and the jejunum has to be done meticulously as leaks in this region will be difficult to manage with percutaneous drainage techniques due to close proximity with the major vessels. Anastomotic leaks in this site can be disastrous, as they behave like proximal small bowel fistulas with severe electrolyte imbalances and result in profound malnutrition. Any deviation from normal postoperative course should alert the surgeon of possible anastomotic leak, and should have a low threshold in evaluating them radiologically. Adding a feeding jejunostomy will add little to the morbidity of the procedure, but might save a patient’s life in case of an unfortunate anastomotic leak. A 5-year survival rate of 50% to 60% with 5-fluorouracil (5-FU) based adjuvant therapy was shown earlier by some authors (9,10). We too offer 5-FU based chemotherapy in patients with adenocarcinoma in adjuvant setup. However, there is not enough data to support the same and is offered as the biology of the tumors seems to be the same of the other GI luminal adenocarcinomas. Two patients that underwent palliative surgery and palliative chemotherapy and one patient with segmental resection for adenocarcinoma have succumbed with a minimum follow up of 8 months. Two patients that had undergone segmental resection followed by adjuvant chemotherapy for adenocarcinoma had no evidence of recurrent disease on follow up at the end of 14 months. Patient with metastatic GIST is on imatinib for disease control and is on follow up at 2 years. There was no evidence of recurrence on follow up imaging in the patient with benign GIST. With the increasing use of advanced imaging methods, these tumors will be identified with increasing frequency and surgeons should be prepared for the difficulties that they are going to face while attempting a curative surgery.
Conclusions
Distal duodenal tumors are increasingly recognized due to advancements in imaging and awareness among surgeons. They can occur in any age group with predominant incidence in male sex. CECT is the investigation of choice to assess operability. Most common presentation is that of a proximal small bowel obstruction. Segmental resection is feasible and may be curative in most of the patients, without the need for vascular resections and reconstructions. Vascular infiltrations pose a problem for curative resection. Adenocarcinomas are the most common histopathological variant. Disease burden in lymph nodes and microscopic vascular invasion on histopathology indicate poor prognosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chung WC, Paik CN, Jung SH, et al. Prognostic factors associated with survival in patients with primary duodenal adenocarcinoma. Korean J Intern Med 2011;26:34-40. [PubMed]
- Hu JX, Miao XY, Zhong DW, et al. Surgical treatment of primary duodenal adenocarcinoma. Hepatogastroenterology 2006;53:858-62. [PubMed]
- Xynopoulos D, Mihas AA, Paraskevas E, et al. Small bowel tumors. Annals of Gastroenterology 2002;15:18-35.
- Koli P, Dewoolkar VV, Butale U. Adenocarcinoma at angle of treitz: a report of two cases with review of literature. Indian J Cancer 2008;45:179-81. [PubMed]
- Markogiannakis H, Theodorou D, Toutouzas KG, et al. Adenocarcinoma of the third and fourth portion of the duodenum: a case report and review of the literature. Cases J 2008;1:98. [PubMed]
- Tocchi A, Mazzoni G, Puma F, et al. Adenocarcinoma of the third and fourth portions of the duodenum: results of surgical treatment. Arch Surg 2003;138:80-5. [PubMed]
- Lowell JA, Rossi RL, Munson JL, et al. Primary adenocarcinoma of third and fourth portions of duodenum. Favorable prognosis after resection. Arch Surg 1992;127:557-60. [PubMed]
- Solej M, D'Amico S, Brondino G, et al. Primary duodenal adenocarcinoma. Tumori 2008;94:779-86. [PubMed]
- North JH, Pack MS. Malignant tumors of the small intestine: a review of 144 cases. Am Surg 2000;66:46-51. [PubMed]
- Howe JR, Karnell LH, Menck HR, et al. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985-1995. Cancer 1999;86:2693-706. [PubMed]