Paraneoplastic cutaneous lupus secondary to esophageal squamous cell carcinoma
Presentation of case
A 59-year-old man with history of alcohol and tobacco use, and hypothyroidism was seen for 3 months history of rash, diarrhea and joint pains.
Patient complained of 3 months history of new symptoms including rash on his extremities and face, and arthralgias in the distal interphalangeal joints, wrists and knees. He reported to have nonbloody loose watery diarrhea occurring 5-7 times a day. He complained of a 30-pound weight loss and night sweats.
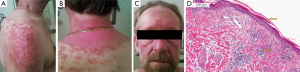
His exam showed annular erythematous scaly papules and plaques involving the shoulders, lower face, neck and forearms (Figure 1A-C). Mild warmth was noted of his wrist and finger joints. Rest of his examination was within normal limits.
Laboratories revealed anemia of chronic disease without evidence of renal insufficiency or liver disease. He had no laboratory evidence of iron deficiency, vitamin B12 or folate deficiency. He had hypothyroidism with thyroid stimulating hormone (TSH) at 8.30 mcIU/mL. His antinuclear antibody (ANA) was positive 1:160 speckled pattern, had negative anti-double stranded DNA and normal complement levels. He had elevated markers of inflammation, C-reactive protein (CRP) at 2.6 mg/dL and erythrocyte sedimentation rate (ESR) at 34 mm/hr. Work up for subacute and chronic diarrhea demonstrated negative serologies for celiac disease, and no evidence of immunoglobulin deficiency. Stool studies were negative for ova and parasites, culture, and C. difficile stool antigen. He had exposure to well-water and stool Giardia antigen was positive. Course of Flagyl yielded transient and incomplete resolution of diarrhea.
Colonoscopy performed for diarrhea of unexplained origin revealed several small and dimunitive hyperplastic polyps and a 6 mm sessile serrated adenoma. A distal colonic villous adenoma was not seen. There was no endoscopic evidence of colitis or ileitis. Random colonic biopsies did not reveal microscopic colitis.
Patient underwent a skin biopsy on the right arm which revealed hyper orthokeratosis, focal parakeratosis, vacuolar alteration and necrotic keratinocytes at dermal epidermal junction with a brisk superficial and deep perivascular lymphocytic inflammatory infiltrate in the dermis (Figure 1D).
Direct immunofluorescence (DIF) examination was unrevealing. These biopsy results along with clinical presentation, physical exam findings, and serologic markers established the diagnosis of subacute cutaneous lupus erythematosus (SCLE).
Contrast enhanced CT of the abdomen and pelvis and chest were obtained to evaluate patient’s weight loss. CT of the abdomen and pelvis revealed innumerable hepatic lesions suspicious for metastases and pathologically enlarged perigastric, para-aortic and retroperitoneal lymph nodes. CT of the chest revealed multiple enlarged mediastinal lymph nodes and two small right lower lobe pulmonary nodules suspicious for pulmonary metastases. Source of origin of metastases was not apparent on these cross-sectional studies.
Tumor markers tested were nonspecific for site of original malignancy. Studies revealed normal AFP at 2.4 ng/mL, moderately elevated CA 19-9 at 156.1 U/mL and CEA at 69.8 ng/mL.
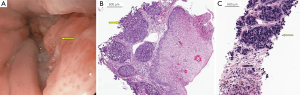
An upper endoscopy was performed to exclude an upper gastrointestinal malignancy. Esophagogastroduodenoscopy (EGD) revealed a 3 cm fungating, nonobstructing, partially circumferential mass in the lower third of the esophagus. Barrett’s mucosa was not observed endoscopically (Figure 2A). Biopsies of the distal esophagus mass revealed an invasive poorly differentiated squamous cell carcinoma (SCC) (Figure 2B). Ultrasound guided percutaneous liver core biopsy confirmed metastatic spread secondary to the esophageal SCC (Figure 2C). Immunoperoxidase stains were positive for cytokeratin-5 and cytokeratin-903 and negative for synaptophysin.
Patient was oriented towards palliative chemotherapy with carboplatin/taxol. Interval imaging showed partial regression of his liver metastases and adenopathy, coinciding with overall clinical improvement in symptoms including improvement in arthritis and rash.
Discussion
Paraneoplastic syndromes comprise of complex set of symptoms and signs that cannot be readily explained by the direct local effect of the tumor or its metastatic spread or by systemic effects of hormones expressed by the tissue from which the tumor arises (1). Paraneoplastic symptoms can be the very first manifestation of an underlying malignancy. As many as 15% of patients diagnosed with a cancer have a concomitant paraneoplastic process at the time of diagnosis (2). Paraneoplastic cutaneous disorders are well described and may be affected by tumor-derived mediators (3). SCLE is a rarely reported paraneoplastic entity and has never been reported in association with squamous cell cancer of the esophagus.
An association between internal malignancy and SCLE was reported in literature since 1980’s. Lung (4), breast, head & neck, gastric, liver, prostate, uterine malignancies and lymphoma association with SCLE have been documented.
Application of McLean’s criteria (5) is useful to identify paraneoplastic dermatosis:
- Onset of dermatosis is after the development of internal malignancy;
- Diagnosis of dermatosis may or may not be made prior to the malignancy diagnosis;
- Malignancy and dermatosis should follow a parallel course;
- Regression of dermatosis once the malignancy is removed and treated.
These criteria help one to establish the temporal proximity of malignancy and paraneoplastic dermatosis.
In our patient, rash and neoplasm followed parallel course. The dermatosis appeared to improve with cytotoxic therapy used to treat the patient’s malignancy.
Diagnosis
SCLE
presence of papulosquamous (33%) and/or annular (97%) lesions on shoulders, forearms, neck and upper torso, absent or mild systemic involvement and presence of anti-Ro (SS-A) antibodies (70%) are the most significant characteristics of SCLE (6). Association with HLA-B8 and HLA-DR3 has been reported (7-10). Approximately 50% of affected patients have systemic lupus erythematosus (SLE), and about 10% of patients with SLE have SCLE (11).
Photosensitivity is twice in prevalence in SCLE when compared to SLE (86% versus 46% respectively) (12). 40-50% of SCLE lesions do not have immune deposits at the dermo-epidermal junction on DIF staining (8). Histopathology is significant for less follicular plugging and hyperkeratosis, absence of dermal atrophy, permanent pigmentary changes and scarring when compared to other cutaneous variants of SLE. Perivascular and peri-appendageal dermal infiltrate is superficial. Vacuolization of the basement membrane and mucin deposition in the dermis occurs. Basement membrane thickening is usually absent or minimal.
Differential diagnosis
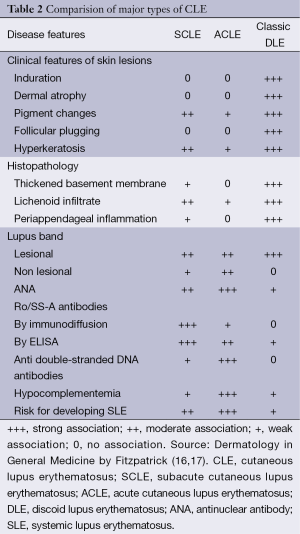
Cutaneous lupus erythematosus (CLE) includes a wide spectrum of skin specific and non specific lesions that would help differentiate the stage of the disease-acute, subacute and chronic. Gilliam and Sontheimer (7,13) classified specific CLE into acute cutaneous LE (ACLE), SCLE and chronic cutaneous LE (CCLE) (Table 1). Comparison of the major types of specific CLE is summarized in Table 2. Papulosquamous SCLE can be confused with psoriasis while annular SCLE can be simulated by erythema multiforme, and figurate erythemas like erythema annulare centrifugum, erythema gyratum repens, and granuloma annulare.

Full table
Full table
Eczemas, seborrheic dermatitis, mycosis fungoides, precancerous conditions like actinic keratosis, papulosquamous disorders like Pityriasis rubra pilaris, connective tissue diseases like Dermatomyositis, Systemic sclerosis, systemic diseases with polyarthralgia like Vasculitis, Still’s disease, Behcet’s disease, viral exanthemata, fungal infections like Tinea corporis, drug eruptions are common mimics of SCLE. A thorough history, comprehensive skin and joint exam, laboratory and pathological work up should be done to confirm and or refute the diagnosis.
Pathological diagnosis
SCLE secondary to metastatic esophageal SCC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mita T, Nakanishi Y, Ochiai A, et al. Paraneoplastic vasculitis associated with esophageal carcinoma. Pathol Int 1999;49:643-7. [PubMed]
- Bojinca V, Janta I. Rheumatic diseases and malignancies. Maedica (Buchar) 2012;7:364-71. [PubMed]
- Szekanecz E, András C, Sándor Z, et al. Malignancies and soluble tumor antigens in rheumatic diseases. Autoimmun Rev 2006;6:42-7. [PubMed]
- Loche F, Schwarze HP, Durieu C, et al. A case of systemic lupus erythematosus associated with cancer of the lung: a paraneoplastic association? Br J Dermatol 2000;143:210-1. [PubMed]
- McLean DI. Cutaneous paraneoplastic syndromes. Arch Dermatol 1986;122:765-7. [PubMed]
- Chlebus E, Wolska H, Blaszczyk M, et al. Subacute cutaneous lupus erythematosus versus systemic lupus erythematosus: diagnostic criteria and therapeutic implications. J Am Acad Dermatol 1998;38:405-12. [PubMed]
- Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981;4:471-5. [PubMed]
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med 1982;97:664-71. [PubMed]
- Sontheimer RD. Subacute cutaneous lupus erythematosus: a decade’s perspective. Med Clin North Am 1989;73:1073-90. [PubMed]
- David-Bajar KM, Bennion SD, DeSpain JD, et al. Clinical, histologic, and immunofluorescent distinctions between subacute cutaneous lupus erythematosus and discoid lupus erythematosus. J Invest Dermatol 1992;99:251-7. [PubMed]
- Wollina U, Barta U, Uhlemann C, et al. Lupus erythematosus-associated red lunula. J Am Acad Dermatol 1999;41:419-21. [PubMed]
- Black DR, Hornung CA, Schneider PD, et al. Frequency and severity of systemic disease in patients with subacute cutaneous lupus erythematosus. Arch Dermatol 2002;138:1175-8. [PubMed]
- Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol 1979;115:1409-15. [PubMed]
- Schur PH, Moschella SL. Mucocutaneous manifestations of systemic lupus erythematosus. Available online: http://www.uptodate.com/contents/mucocutaneous-manifestations-of-systemic-lupus-eryth
- Moschella SL, Hurley HJ. eds. Moschella’s Dermatology. Moschella and Hurley Dermatology. 3rd ed. WB Saunders Co: Philadelphia, 1992:1222-6.
- Freedberg IM, Eisen AZ, Wolff K, et al. eds. Fitzpatrick's Dermatology in General Medicine. 5th edition. USA, New York: The McGraw-Hill Companies, 1999:1997-2007.
- Provost TT. The relationship between discoid and systemic lupus erythematosus. Arch Dermatol 1994;130:1308-10. [PubMed]


