
Barrett’s esophagus: A review of diagnostic criteria, clinical surveillance practices and new developments
The current definition for Barrett’s esophagus (BE) proposed by the American Gastroenterological Association (AGA) is “the condition in which any extent of metaplastic columnar epithelium that predisposes to cancer development replaces the stratified squamous epithelium that normally lines the distal esophagus (1)”. Three types of columnar epithelium are seen in the setting of BE: (I) gastric-fundic type, (II) cardia-type, and (III) intestinal-type including goblet cells. However, only the last type has been clearly linked to an increased risk of malignant progression, with a reported annual risk of esophageal adenocarcinoma (EAC) of about 0.5% per year in patients with intestinal metaplasia of the esophagus (1-3). For this reason both the AGA and the American College of Gastroenterology (ACG) currently recommend that although columnar-type mucosa can be recognized during endoscopy, the presence of intestinal metaplasia must be confirmed by biopsy before rendering a diagnosis of BE (1,4).
Controversies regarding intestinal metaplasia
The American definition is used in most parts of the world, however, Great Britain and Japan allow the diagnosis of BE to be assigned if only cardiac-type metaplasia is seen on biopsy (5,6). While some advocate the universal adoption of the less stringent criteria (7), the evidence to do so is controversial. Gatenbyet al. and Keltyet al. each conducted studies that showed a similar risk of EAC in patients having columnar metaplasia of the esophagus with and without goblet cells (8,9). In contrast, two large population studies from Northern Ireland showed a clear increased risk of cancer when intestinal metaplasia was present versus when only columnar cell change was identified (10,11).
A study by Takuboet al. which examined the mucosa adjacent to EAC treated with endoscopic mucosal resection found that most (>70%) were bordered by cardiac-type mucosa rather than intestinal-type mucosa and that 56% had no intestinal-type mucosa in any areas of the resection specimens. They concluded that there is a relationship between EAC and cardiac-type mucosa and that a background of intestinal metaplasia may not be a necessary pre-requisite to EAC (6).
Two similar studies by Chandrasoma and colleagues had different findings. The first, which examined esophagogastrectomy specimens resected due to adenocarcinoma, showed cardiac mucosa adjacent to all tumors but also showed residual intestinal metaplasia in 65% of cases overall and in 100% of intramucosal tumors as well as those less than 1 cm in diameter (12). The second study reviewed two groups: (I) cases with visible columnar metaplasia of the esophagus which underwent systematic 4-quadrant biopsies every 1 to 2 cm and (II) cases of dysplasia or EAC which did not receive systematic biopsy. They found that when systematic biopsy was performed, intestinal metaplasia was identified in >87% of cases including all cases with dysplasia or EAC. None of the cases with cardiac type epithelium alone had dysplasia or EAC. In the group which did not receive systematic biopsy but did have dysplasia or EAC, many showed only tumor on biopsy. However, slightly more than half (56%) of those with non-tumor mucosa had residual intestinal-type metaplasia (13). They hypothesize that the absence of residual intestinal metaplasia immediately adjacent to many cases of EAC is due to tumor overgrowth and inadequate sampling rather than a true absence (12,13). They also propose that when metaplastic columnar epithelium is adequately and systematically biopsied, patients without intestinal metaplasia have a negligible risk of dysplasia and cancer (13).
Recent data shows that columnar cell epithelium may have an intestinal-type immunohistochemical profile even when goblet cells are not identified. Various studies have shown significantly increased positivity for intestinal markers such as DAS-1 (14-16), CDX-2 (14,17,18), and HepPar1 (19) as well as a similar cytokeratin (16,20) and mucin (20) expression profile in both goblet cell and non-goblet cell columnar epithelia, which suggests a similar origin. There have also been studies showing similar molecular alterations in both non-goblet cell and intestinal-type metaplasia including chromosomal instability (21,22), microsatellite instability (22), and similar DNA content abnormalities (23). Despite the similar phenotypic and molecular profiles, the natural history of columnar cells and goblet cells is not always the same (24) suggesting that additional factors are required for progression toward dysplasia and cancer.
Expanding the definition of BE to include all patients with columnar metaplasia of the esophagus would have substantial societal and personal economic impact. Studies from both the United States and Sweden show that the population of patients with columnar metaplasia of the esophagus without goblet cells is significantly greater than the population with intestinal metaplasia (25,26). Conducting surveillance on all of these patients has the potential to overwhelm healthcare resources and greatly increase treatment costs. Also, despite data which demonstrate a normal life expectancy in patients with BE, the cost of life insurance is substantially increased and availability of health insurance is decreased in patients with this diagnosis (27). Until such a time as columnar cell metaplasia of the esophagus without goblet cells is clearly shown to convey increased risk of EAC, it seems appropriate to hold back from labeling these patients with BE (1,4).
Screening
Endoscopic screening for BE is widely practiced and patients are often selected for screening based on the presence of multiple well-established risk factors for BE including: chronic gastroesophageal reflux disease (GERD), older age (>50 years), male sex, white race, elevated body mass index, intra-abdominal fat distribution, and hiatal hernia (1,4).
While the presence of GERD symptoms was one of the first recognized and strongest risk factors identified for BE, the presence of GERD alone is not sufficient to recommend screening. Up to 40% of US adults experience GERD on a monthly basis (28), yet despite the increasing incidence of EAC there are still fewer than 10,000 new cases of EAC diagnosed per year (29). Up to 40% of patients who have adenocarcinoma of the esophagus report no history of chronic GERD (30). Eliminating patients from screening based on a lack of symptoms could exclude a large portion of those who might have their cancers detected at an early, presymptomatic stage. Additionally, difficulties recognizing mucosal lesions (31),sampling error (32), and disagreement over pathologic interpretation (33) can decrease the effectiveness of endoscopic screening. For these reasons, the decision of who and when to screen should be individualized (1,4).
Endoscopic diagnosis
Barrett’s esophagus (BE) presents on endoscopy as characteristic salmon-pink colored extensions (or “tongues”) of mucosa that grow into the esophagus above the esophageal gastric junction (EGJ). For screening and surveillance, four quadrant biopsies are taken along every 2 cm of the BE type mucosa and submitted to pathology in separate containers. While this approach samples only a small fraction of the affected lining, it allows the opportunity to recognize dysplasia and focus subsequent biopsies on the appropriate area if dysplasia is identified (4). Traditionally, BE is termed long segment if the tongues are 3 cm or more in length, short segment BE when less than 3 cm, and ultra-short segment BE when less than 1 cm (34). The exact location of the biopsy relative to the Z-line and EGJ is important to know, as ultra-short BE can be difficult to differentiate from an irregular EGJ and is thought to carry significantly less risk of cancer development than traditional BE (34-38). Additionally, intestinal metaplasia below the EGJ should not be diagnosed as BE. The changes are thought to have a different etiology, often arising secondary to Helicobacter pylori infection, and the significance as a precursor to EAC is uncertain (35,39-41). For these reasons, changes in this region should be given a descriptive diagnosis of intestinal metaplasia.
Accurate assessment of the extent of BE on endoscopy is clinically important because more extensive BE carries a higher risk of cancer development (42,43), however there is a high degree of inter- and intraobserver variation (44-46). The Prague C&M Criteria (47) is a consensus-driven, validated system which utilizes standardized landmarks - thesquamocolumnar junction, the EGJ, the extent of circumferential columnar lining, and the proximal extension of the columnar mucosa - to determine the length of BE. This system has an overall reliability coefficient (RC) of 0.72 in recognition of BE ≥1 cm, however the RC dropped to 0.22 when less than 1 cm of columnar-lining was present. This is the endoscopic classification system currently suggested by the American College of Gastroenterology (4). A recent small study by Kinjo et al. (48) suggests that recognition of ultra-short segment BE may be improved using the Japanese EGJ reference point (the distal end of the palisade-shaped longitudinal vessels) rather than the traditional proximal limit of the linear gastric mucosal folds currently utilized in the Prague C&M criteria, but more information is needed to determine if these results are reproducible and applicable outside of the Japanese population.
Histologic features of Barrett’s esophagus and dysplasia
Clinicians and pathologists have defined BE to include not only a characteristic endoscopic appearance to the esophagus but also histologically confirmed intestinal metaplasia consisting of columnar epithelium with well-formed goblet cells (1). Goblet cells are recognized by a large cytoplasmic vacuole filled with blue-tinted mucin. During carcinogenesis, the tissue develops morphologic changes related to unregulated cell growth that can be recognized as dysplasia on microscopic examination (49). The spectrum of changes is subdivided into four clinically significant groups: negative for dysplasia, indefinite for dysplasia, low grade dysplasia, and high grade dysplasia. Patients with histologically confirmed dysplasia have been shown to have significantly increased risk of progression to EAC (33,50-52). Despite concerns over adequate sampling and imperfect intra- and interobserver reproducibility (particularly at the low end of the dysplasia spectrum), histologic evaluation for dysplasia retains a key role in the surveillance of patients with BE (4,33,53).
Due to the significance of identifying dysplasia, much work has gone into clarifying and refining the criteria used to interpret biopsies (33,54-57). The degree of dysplasia is determined by evaluating the cytology (nuclear and cytoplasmic features), architecture (relationship of glands and lamina propria), and degree of surface maturation (comparison of nuclear size within crypts to nuclear size at the mucosal surface) and interpreting these findings in conjunction with the amount of background inflammation. Features of each category of dysplasia are described below and summarized in Table 1.
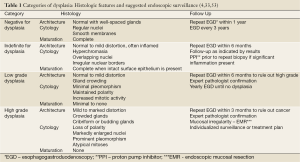
Full table
Negative for dysplasia – These biopsies can have a minimal amount of cytologicatypia but retain normal architecture, abundant lamina propria between glands, and appropriate maturation with a low nuclear:cytoplasmic ratio at the mucosal surface. The nuclei are regular, have smooth membranes, and are basally situated. If mitoses are present they are within the basal compartment. In the presence of inflammation, increased cytologicatypia is allowed (Figure 1A).
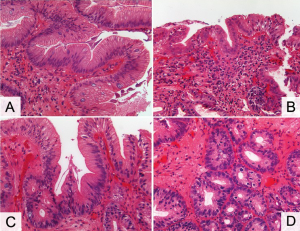
Indefinite for dysplasia – This category is applied to biopsies where the changes seen cannot be definitively described as reactive or neoplastic. It is most often used in the presence of pronounced inflammation or the loss of surface epithelium. Cytologicatypia characterized by hyperchromasia, overlapping nuclei, irregular nuclear borders, and nuclear stratification can be seen in the deep glands or the sides of villiform structures while the surface epithelium is free of atypia. The architecture should be largely normal with, at the most, minimal gland crowding. Surface maturation is present (Figure 1B).
Low grade dysplasia – The most important feature of low grade dysplasia is cytologicatypia extending to the mucosal surface and either minimal or absent surface maturation. Severe architectural distortion is not a feature, though mild gland crowding with decreased intervening lamina propria can be seen. Mitoses may be increased but no atypical forms should be seen. Inflammation is usually minimal. One important note: although cytologicatypia is a key finding, nuclear polarity is preserved. Loss of polarity - where the nucleus is tilted, rounded, or horizontal to the basement membrane - is associated with higher grade lesions (Figure 1C).
High grade dysplasia – The cytologic changes are severe with markedly enlarged nuclei at the surface, pronounced pleomorphism, and at least focal loss of nuclear polarity. Surface maturation is lost. Mild to marked architectural distortion is a frequent finding, with crowded glands, loss of lamina propria, focal budding, and/or cribriform glands. There should be no evidence of invasion into the lamina propria. Mitoses are increased and atypical mitoses may be seen. Ideally inflammation is minimal or absent. If either the cytologic or architectural changes are severe and extensive, the diagnosis of high grade dysplasia can be made even if other features are only low grade in severity (Figure 1D).
Whenever high grade dysplasia is diagnosed the biopsy should also be evaluated for the presence of co-existing EAC. This may be difficult or impossible to exclude on biopsy, but suspicious or suggestive architectural changes include single cells in the lamina propria, desmoplasia, cribriform or solid tubular architecture, dilated tubules filled with necrotic debris, extensive neutrophilic infiltrate within the epithelium, ulcerated high grade dysplasia, and neoplastic tubules incorporated into the overlying squamous epithelium (57).
Surveillance of Barrett’s esophagus
Although there is a lack of randomized trials that support the value of endoscopic surveillance in BE, indirect evidence from multiple retrospective studies indicates a statistically significant improvement in survival for cancers that are detected endoscopically versus those that present with symptoms (58-64). In light of this evidence and the poor 5 year survival for EAC, surveillance endoscopy is widely practiced (65,66).
Ideally surveillance endoscopy is performed in patients whose reflux symptoms are controlled, reducing the chance of inflammatory or reactive changes interfering with pathologic interpretation (67). Four quadrant biopsies should be obtained at a minimum of every 2 cm and submitted to pathology in separate containers. The surveillance intervals suggested by the 2008 ACG Guidelines (4) are dependent on the pathology results (Table 1).
If the initial biopsy diagnostic of BE is negative for dysplasia, a repeat endoscopic exam with biopsy is recommended within a year. If the second study is also negative for dysplasia then follow-up at 3 year intervals is suggested. If low grade dysplasia is identified it is suggested that the diagnosis be confirmed by second opinion from an expert pathologist and a repeat exam take place within 6 months to ensure no higher grade of dysplasia is identified. If no higher grade lesion is found, yearly follow up is suggested until two consecutive exams are negative for dysplasia. Biopsies interpreted as indefinite for dysplasia should be managed similarly to those with low grade dysplasia. A diagnosis of high grade dysplasia should also be confirmed by an expert pathologist but repeat exam should take place within 3 months. Biopsies should be taken at smaller, 1 cm intervals. It is also suggested that any mucosal irregularities be treated with endoscopic mucosal resection to obtain enough tissue for accurate diagnosis. Beyond these suggestions, treatment options for high-grade dysplasia include careful surveillance, a variety of ablative therapies, and surgical resection. Treatment should be tailored for individual patients based on their preferences, their appropriateness for each option, and the experience of the treating physician (4).
Developments in the diagnosis and surveillance of Barrett’s esophagus
Controversies over the best methods to diagnosis and monitor BE exist, largely because the current process involves many variables that are subjective and therefore difficult to standardize: selection of patients for screening, recognition of landmarks and BE-type changes on endoscopy, sampling variation, histologic grading of dysplasia, and the timing and type of intervention. The ultimate goal is to detect cancers that develop in the setting of BE at a curable stage. Advances in techniques are being explored, with most of the emphasis placed either on increasing the recognition of suspicious lesions for biopsy during endoscopy or objectively identifying which cases of dysplasia are likely to progress to carcinoma using biomarkers.
Enhanced endoscopy
High-resolution white light endoscopy combines endoscopes with large numbers of pixels (600,000 to 1,000,000), magnification devices, and high-definition screens to optimize visualization of the esophageal mucosa (68).It has shown greater sensitivity in the detection of early neoplastic lesions when compared to standard endoscopy (69).
Chemoendoscopy involves the application of chemicals that selectively react with and highlight various mucosal features, theoretically improving the detection of abnormalities (70-76). Methylene blue is absorbed by non-dysplastic intestinal-type epithelial cells theoretically helping to detect BE and target biopsies. However, meta-analysis found no significant difference in the detection rates of BE or dysplasia between methylene blue directed biopsy and a standard 4-quadrant approach (74). Additonally, there is some evidence that methylene blue induces DNA damage in BE (77,78). Lugol’s solution is absorbed by glycogen-containing squamous epithelial cells and helps identify the squamocolumnar junction after eradication therapy, which is helpful in the recognition of residual columnar-cell islands (75). Indigo carmine dye is combined with magnification endoscopy to distinguish mucosal pit patterns - round, reticular, villous, and ridged (68). While there is good association of certain patterns with intestinal metaplasia (76), it has not been shown to increase the detection of dysplasia beyond that of high-resolution endoscopy (69).
Electronic chromoendoscopy includes optimal band imaging which involves postprocessing to accentuate the contrast between columnar and squamous epithelia (79) and narrow band imaging (NBI) which uses optical filters to highlight vascular patterns on the mucosal surface (80). While studies show good correlation of vascular patterns identified by magnified NBI with BE and high grade dysplasia (80,81), prospective studies comparing the actual diagnostic yield of NBI to standard endoscopy have had mixed results (82-84). A comparison of NBI to high resolution white light endoscopy showed no significant difference in the detection of BE or dysplasia (84).
Autofluorescence imaging utilizes differences in the endogenous fluorophores found in normal and neoplastic epithelia (68). While the technique has good sensitivity for the detection of high grade dysplasia, studies have shown poor specificity with false positive rates up to 81% (85-87). An analogous process recently described by Bird-Lieberman et al. utilizes a fluorescently labeled wheat germ derived lectin that binds to surface glycans of normal esophageal epithelial cells. Expression of these glycans is decreased or lost during neoplastic progression, so potentially pre-malignant or malignant regions are highlighted by a negative staining pattern (88). The potential applications are intriguing, but it has yet to be applied in vivo or prospective clinical trial.
Magnifications exceeding 1,000× can be achieved in real time using confocal laser endomicroscopy, allowing for analysis of the crypt architecture and capillaries during endoscopic examination. A few initial studies have shown accuracy rates above 85% in detecting high-grade dysplasia (89,90), fused glands indicating neoplasia with a sensitivity of 80%, and good interobserver agreement (91).
Light scattering spectroscopy and diffuse reflectance spectroscopy use algorithms to analyze light scattered back to the sensing device by the tissue. This spectroscopic information has been able to distinguish neoplastic from non-neoplastic tissue with both good sensitivity and specificity (92,93) in a few small trials. Optical coherence tomography uses variations in the reflectance of near-infrared light from different tissues to create a high-resolution cross-sectional image of the mucosa (94). One study has shown excellent sensitivity (97%) and specificity (92%) in the recognition of BE without dysplasia (95) while another showed good sensitivity (83%) and specificity (75%) in identifying high grade dysplasia (96).
While many of these endoscopic techniques show promise, there is currently no definitive evidence that they provide additional information beyond careful examination using high-resolution white light endoscopy. Also, most require specialized equipment and/or training that may not currently be available outside of specialty centers.
Biomarkers
The grading of dysplasia currently guides surveillance and treatment decisions; however it is an imperfect predictor of cancer risk. Several biomarkers have shown promise as objective adjunct tests to improve risk stratification of patients with BE. Panels of immunohistochemical stains including α-methylacyl-CoA racemase (AMACR), β-catenin, cyclin D1, and p53 show promise in separating grades of dysplasia and in distinguishing true neoplastic progression from reactive changes (97-99).
Other biomarkers which test for DNA abnormalities have been evaluated in cross-sectional or retrospective studies. The detection of aneuploidy, increased tetraploidy, and loss of heterozygosity (LOH) for chromosome 17p in patients with no dysplasia or low grade dysplasia on biopsy has been shown to have good predictive value for neoplastic progression, but added little information when high grade dysplasia was detected (100-104). These studies utilized flow cytometry to detect DNA content abnormalities in fresh frozen tissue, which may not be practical in clinical practice. Fluorescence in situ hybridization can theoretically be used to detect these same abnormalities in fixed tissue and most initial studies show promising results (104-108).
Biomarker panels - including detection of chromosomal abnormalities (aneuploidy/tetraploidy, 17p LOH, 9p LOH) or tumor-suppressor gene-methylation patterns - have also been good indicators of progression risk in initial studies (109-111). One methylation-based panel, applied retrospectively, even identified patients who progressed to high grade dysplasia two years before histologic changes were seen (111).
Biomarkers may prove to be the best predictors of cancer progression, however much of the work done with them to-date has involved freshly frozen tissue (which may not always be available) and none have been validated in prospective controlled clinical trials. While promising, they should not replace grading dysplasia for risk stratification in routine clinical practice at this time (68).
Conclusions
Although newer techniques are being studied, at this time none have definitively been shown to be more cost effective than careful clinical evaluations and systematic biopsy screening. Good patient care includes coordination of careful microscopic study with patient clinical history. The findings of both the endoscopist and the pathologist are critical.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084-91.
- Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2010;8:235-44. [PubMed]
- Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol 2008;168:237-49. [PubMed]
- Wang KK, Sampliner REPractice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol 2008;103:788-97. [PubMed]
- British Society of Gastroenterology. Guidelines for the diagnosis and management of Barrett’s columnar lined oesophagus. A report of the working party of the British Society of Gastroenterology. [updated 2009; cited 2012 March 28]. Available online: http://www.bsg.org.uk
- Takubo K, Aida J, Naomoto Y, et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum Pathol 2009;40:65-74. [PubMed]
- Riddell RH, Odze RD. Definition of Barrett’s esophagus: time for a rethink--is intestinal metaplasia dead? Am J Gastroenterol 2009;104:2588-94. [PubMed]
- Gatenby PA, Ramus JR, Caygill CP, et al. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol 2008;43:524-30. [PubMed]
- Kelty CJ, Gough MD, VanWyck Q, et al. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol 2007;42:1271-4. [PubMed]
- Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst 2011;103:1049-57. [PubMed]
- Murray L, Watson P, Johnston B, et al. Risk of adenocarcinoma in Barrett’s oesophagus: population based study. BMJ 2003;327:534-5. [PubMed]
- Chandrasoma P, Wickramasinghe K, Ma Y, et al. Is intestinal metaplasia a necessary precursor lesion for adenocarcinomas of the distal esophagus, gastroesophageal junction and gastric cardia? Dis Esophagus 2007;20:36-41. [PubMed]
- Chandrasoma P, Wijetunge S, DeMeester S, et al. Columnar-lined esophagus without intestinal metaplasia has no proven risk of adenocarcinoma. Am J Surg Pathol 2012;36:1-7. [PubMed]
- Hahn HP, Blount PL, Ayub K, et al. Intestinal differentiation in metaplastic, nongoblet columnar epithelium in the esophagus. Am J Surg Pathol 2009;33:1006-15. [PubMed]
- Rogge-Wolf C, Seldenrijk CA, Das KM, et al. Prevalence of mabDAS-1 positivity in biopsy specimens from the esophagogastric junction. Am J Gastroenterol 2002;97:2979-85. [PubMed]
- DeMeester SR, Wickramasinghe KS, Lord RV, et al. Cytokeratin and DAS-1 immunostaining reveal similarities among cardiac mucosa, CIM, and Barrett’s esophagus. Am J Gastroenterol 2002;97:2514-23. [PubMed]
- Phillips RW, Frierson HF Jr, Moskaluk CA. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol 2003;27:1442-7. [PubMed]
- Steininger H, Pfofe DA, Muller H, et al. E Expression of CDX2 and MUC2 in Barrett’s mucosa. Pathol Res Pract 2005;201:573-7. [PubMed]
- Chu PG, Jiang Z, Weiss LM. Hepatocyte antigen as a marker of intestinal metaplasia. Am J Surg Pathol 2003;27:952-9. [PubMed]
- Glickman JN, Chen YY, Wang HH, et al. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett’s esophagus. Am J Surg Pathol 2001;25:569-78. [PubMed]
- Chaves P, Crespo M, Riberio C, et al. Chromosomal analysis of Barrett’s cells: demonstration of instability and detection of the metaplastic lineage involved. Mod Pathol 2007;20:788-96. [PubMed]
- Romagnoli S, Roncalli M, Graziani D, et al. Molecular alterations of Barrett’s esophagus on microdissected endoscopic biopsies. Lab Invest 2001;81:241-7. [PubMed]
- Liu W, Hahn H, Odze RD, et al. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol 2009;104:816-24. [PubMed]
- Horwhat JD, Baroni D, Maydonovitch C, et al. Normalization of intestinal metaplasia in the esophagus and esophagogastric junction: incidence and clinical data. Am J Gastroenterol 2007;102:497-506. [PubMed]
- Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670-7. [PubMed]
- Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology 2005;129:1825-31. [PubMed]
- Shaheen NJ, Dulai GS, Ascher B, et al. Effect of a new diagnosis of Barrett’s esophagus on insurance status. Am J Gastroenterol 2005;100:577-80. [PubMed]
- Locke GR 3rd, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997;112:1448-56. [PubMed]
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005;97:142-6. [PubMed]
- Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31. [PubMed]
- Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol 1999;94:937-43. [PubMed]
- Chatelain D, Fléjou JF. High-grade dysplasia and superficial adenocarcinoma in Barrett’s esophagus: histological mapping and expression of p53, p21 and Bcl-2 oncoproteins. Virchows Arch 2003;442:18-24. [PubMed]
- Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368-78. [PubMed]
- Mueller J, Werner M, Stolte M. Barrett’s esophagus: histopathologic definitions and diagnostic criteria. World J Surg 2004;28:148-54. [PubMed]
- Goldblum JR. The significance and etiology of intestinal metaplasia of the esophagogastric junction. Ann Diagn Pathol 2002;6:67-73. [PubMed]
- Spechler SJ. Short and ultrashort Barrett’s esophagus -- what does it mean. Semin Gastrointest Dis 1997;8:59-67. [PubMed]
- Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1998;93:1028-32. [PubMed]
- Weston AP, Krmpotich PT, Cherian R, et al. Prospective long-term endoscopic and histological follow-up of short segment Barrett’s esophagus: comparison with traditional long segment Barrett’s esophagus. Am J Gastroenterol 1997;92:407-13. [PubMed]
- Chang Y, Liu B, Liu GS, et al. Short-segment Barrett’s esophagus and cardia intestinal metaplasia: A comparative analysis. World J Gastroenterol 2010;16:6151-4. [PubMed]
- Goldblum JR, Richter JE, Vaezi M, et al. Helicobacter pylori infection, not gastroesophageal reflux, is the major cause of inflammation and intestinal metaplasia of gastric cardiac mucosa. Am J Gastroenterol 2002;97:302-11. [PubMed]
- Wijetunge S, Ma Y, DeMeester S, et al. Association of adenocarcinoma of the distal esophagus, “gastroesophageal junction,” and “gastric cardia” with gastric pathology. Am J SurgPathol 2010;34:1521-7.
- Gopal DV, Lieberman DA, Magaret N, et al. Risk factors for dysplasia in patients with Barrett’s esophagus (BE): results from a multi-center consortium. Dig Dis Sci 2003;48:1537-41. [PubMed]
- Menke-Pluymers MB, Hop WC, Dees J, et al. Risk factors for the development of an adenocarcinoma in columnar-lined (Barrett) esophagus. The Rotterdam Esophageal Tumor Study Group. Cancer 1993;72:1155-8. [PubMed]
- Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus--the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033-6. [PubMed]
- Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994;107:945-9. [PubMed]
- Dekel R, Wakelin DE, Wendel C, et al. Progression or regression of Barrett’s esophagus--is it all in the eye of the beholder? Am J Gastroenterol 2003;98:2612-5. [PubMed]
- Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392-9. [PubMed]
- Kinjo T, Kusano C, Oda I, et al. Prague C & M and Japanese criteria: shades of Barrett’s esophagus endoscopic diagnosis. J Gastroenterol 2010;45:1039-44. [PubMed]
- Spechler SJ. Dysplasia in Barrett’s esophagus: limitations of current management strategies. Am J Gastroenterol 2005;100:927-35. [PubMed]
- Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol 2000;95:3383-7. [PubMed]
- Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394-98. [PubMed]
- Montgomery E, Goldblum JR, Greenson JK, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol 2001;32:379-88. [PubMed]
- Voltaggio L, Montgomery EA, Lam-Himlin D. A clinical and histopathologic focus on Barrett esophagus and Barrett-related dysplasia. Arch Pathol Lab Med 2011;135:1249-60. [PubMed]
- Theisen J, Nigro JJ, DeMeester TR, et al. Chronology of the Barrett’s metaplasia-dysplasia-carcinoma sequence. Dis Esophagus 2004;17:67-70. [PubMed]
- Cameron AJ, Carpenter HA. Barrett’s esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol 1997;92:586-91. [PubMed]
- Reid BJ, Haggitt RC, Rubin CE, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol 1988;19:166-78. [PubMed]
- Zhu W, Appelman HD, Greenson JK, et al. A histologically defined subset of high-grade dysplasia in Barrett mucosa is predictive of associated carcinoma. Am J Clin Pathol 2009;132:94-100. [PubMed]
- Streitz JM Jr, Andrews CW Jr, Ellis FH Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac CardiovascSurg 1993;105:383-7. [PubMed]
- Peters JH, Clark GW, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac CardiovascSurg 1994;108:813-21. [PubMed]
- van Sandick JW, van Lanschot JJ, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut 1998;43:216-22. [PubMed]
- Incarbone R, Bonavine L, Saino G, et al. Outcome of esophageal adenocarcinoma detected during endoscopic biopsy surveillance for Barrett’s esophagus. Surg Endosc 2002;16:263-6. [PubMed]
- Ferguson MK, Durkin A. Long-term survival after esophagectomy for Barrett’s adenocarcinoma in endoscopically surveyed and nonsurveyed patients. J Gastrointest Surg 2002;6:29-35. [PubMed]
- Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004;91:997-1003. [PubMed]
- Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology 2002;122:633-40. [PubMed]
- Falk GW, Ours TM, Richter JE. Practice patterns for surveillance of Barrett’s esophagus in the United States. Gastrointest Endosc 2000;52:197-203. [PubMed]
- Gross CP, Canto MI, Hixson J, et al. Management of Barrett’s esophagus: a national study of practice patterns and their cost implications. Am J Gastroenterol 1999;94:3440-7. [PubMed]
- Hanna S, Rastogi A, Weston AP, et al. Detection of Barrett’s esophagus after endoscopic healing of erosive esophagitis. Am J Gastroenterol 2006;101:1416-20. [PubMed]
- Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011;140:e18-52. [PubMed]
- Kara MA, Peters FP, Rosmolen WD, et al. High-resolution endoscopy plus chromoendoscopy or narrow-band imaging in Barrett’s esophagus: a prospective randomized crossover study. Endoscopy 2005;37:929-36. [PubMed]
- Canto MI, Setrakian S, Willis JE, et al. Methylene blue staining of dysplastic and nondysplastic Barrett’s esophagus: an in vivo and ex vivo study. Endoscopy 2001;33:391-400. [PubMed]
- Breyer HP, Silva De Barros SG, Maguilinik I, et al. Does methylene blue detect intestinal metaplasia in Barrett’s esophagus? Gastrointest Endosc 2003;57:505-9. [PubMed]
- Horwhat JD, Maydonovitch CL, Ramos F, et al. A randomized comparison of methylene blue-directed biopsy versus conventional four-quadrant biopsy for the detection of intestinal metaplasia in patients with long-segment Barrett’s esophagus. Am J Gastroenterol 2008;103:546-54. [PubMed]
- Guelrud M, Herrera I. Acetic acid improves identification of remnant islands of Barrett’s epithelium after endoscopic therapy. Gastrointest Endosc 1998;47:512-5. [PubMed]
- Ngamruengphong S, Sharma VK, Das A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc 2009;69:1021-8. [PubMed]
- Guelrud M, Herrera I, Essenfeld H, et al. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett’s esophagus. Gastrointest Endosc 2001;53:559-65. [PubMed]
- Sharma P, Weston AP, Topalvoski M, et al. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett’s oesophagus. Gut 2003;52:24-7. [PubMed]
- Davies J, Burke D, Oliver JR, et al. Methylene blue but not indigo carmine causes DNA damage to colonocytes in vitro and in vivo at concentrations used in clinical chromendoscopy. Gut 2007;56:155-6. [PubMed]
- Olliver JR, Wild CP, Sahay P, et al. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet 2003;362:373-74. [PubMed]
- Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt 2004;9:568-77. [PubMed]
- Kara MA, Ennahachi M, Fockens P, et al. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointest Endosc 2006;64:155-66. [PubMed]
- Sharma P, Bansal A, Mathur S, et al. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest Endosc 2006;64:167-75. [PubMed]
- Curvers W, Baak L, Kiesslick R, et al. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus. Gastroenterology 2008;134:670-9. [PubMed]
- Wolfsen HC, Crook JE, Krishna M, et al. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s esophagus. Gastroenterology 2008;135:24-31. [PubMed]
- Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international randomized controlled trial. Gut 2012; [Epub ahead of print]. [PubMed]
- Kara MA, Peters FP, Ten Kate FJ, et al. Endoscopic video Autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest Endosc 2005;61:679-85. [PubMed]
- Curvers WL, Herrero LA, Wallace MB, et al. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett’s esophagus. Gastroenterology 2010;139:1106-14. [PubMed]
- Curvers WL, Singh R, Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut 2008;57:167-72. [PubMed]
- Bird-Lieberman EL, Neves AA, Lao-Sirieix P, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat Med 2012;18:315-21. [PubMed]
- Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol 2006;4:979-87. [PubMed]
- Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasia and colorectal cancer in vivo. Gastroenterology 2004;127:706-13. [PubMed]
- Pohl H, Rosch T, Vieth M, et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett’s oesophagus. Gut 2008;57:1648-53. [PubMed]
- Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with Barrett’s esophagus. Gastroenterology 2001;120:1620-9. [PubMed]
- Wallace MB, Perelman LT, Backman V, et al. Endoscopic detection of dysplasia in patients with Barrett’s esophagus using light-scattering spectroscopy. Gastroenterology 2000;119:677-82. [PubMed]
- Kobayashi K, Izatt JA, Kulkarni MD, et al. High-resolution cross-sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc 1998;47:515-23. [PubMed]
- Poneros JM, Brand S, Bouma BE, et al. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology 2001;120:7-12. [PubMed]
- Evans JA, Poneros JM, Bouma BE, et al. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol 2006;4:38-43. [PubMed]
- van Dekken H, Hop WC, Tilanus HW, et al. Immunohistochemical evaluation of a panel of tumor cell markers during malignant progression in Barrett esophagus. Am J Clin Pathol 2008;130:745-53. [PubMed]
- Dorer R, Odze RD. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus, ulcerative colitis, and Crohn’s disease. Am J Surg Pathol 2006;30:871-7. [PubMed]
- Shi XY, Bharwandeen B, Leong AS. p16, cyclin D1, Ki-67, and AMACR as markers for dysplasia in Barrett esophagus. Appl Immunohistochem Mol Morphol 2008;16:447-52. [PubMed]
- Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol 2000;95:1669-76. [PubMed]
- Rabinovitch PS, Longton G, Blount PL, et al. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am J Gastroenterol. 2001;96:3071-83. [PubMed]
- Kerkhof M, Steyerberg EW, Kusters JG, et al. Aneuploidy and high expression of p53 and Ki67 is associated with neoplastic progression Barrett esophagus. Cancer Biomark 2008;4:1-10. [PubMed]
- Reid BJ, Prevo LJ, Galipeau PC, et al. Predictors of progression in Barrett’s esophagus II: baseline 17p (p53) loss of heterozygosity identified a patient subset at increased risk for neoplastic progression. Am J Gastroenterol 2001;96:2839-48. [PubMed]
- Rygiel AM, Milano F, Ten Kate FJ, et al. Assessment of chromosomal gains as compared to DNA content changes is more useful to detect dysplasia in Barrett’s esophagus brush cytology specimens. Genes Chromosomes Cancer 2008;47:396-404. [PubMed]
- Fahmy M, Skacel M, Gramllich TL, et al. Chromosomal gains and genomic loss of p53 and p16 in Barrett’s esophagus detected by fluorescence in situ hybridization of cytology specimens. Mod Pathol 2004;17:588-96. [PubMed]
- Wongsurawat VJ, Finley JC, Galipeau PC, et al. Genetic mechanisms of TP53 loss of heterozygoisty in Barrett’s esophagus: implications for biomarker validation. Cancer Epidemiol Biomarkers Prev 2006;15:509-16. [PubMed]
- Cestari R, Villanacci V, Rossi E, et al. Fluorescence in situ hybridization to evaluate dysplasia in Barrett’s esophagus: a pilot study. Cancer Lett 2007;251:278-87. [PubMed]
- Rygiel AM, van Baal JW, Milano F, et al. Efficient automated assessment of genetic abnormalities detected by fluorescence in situ hybridization on brush cytology in a Barrett esophagus surveillance population. Cancer 2007;109:1980-8. [PubMed]
- Galipeau PC, Li X, Blount PL, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med 2007;4:e67. [PubMed]
- Jin Z, Cheng Y, Gu W, et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res 2009;69:4112-5. [PubMed]
- Schulmann K, Sterian A, Berki A, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene 2005;24:4138-48. [PubMed]


