Gastrointestinal stromal tumor with an unusual presentation as an enlarged prostate gland: a case report and review of the literature
Introduction
Primary prostatic gastrointestinal stromal tumor (GIST) is an extremely rare entity that may present with non-specific urinary symptoms (1,2). To the best of our knowledge, there have been only five reported cases of primary prostatic GISTs (1-5). While the vast majority of GISTs occur within the gastrointestinal tract [the stomach is the most frequent site (6)], tumors arising from other locations have been rarely reported, including the retroperitoneum, mesentery, and omentum (5). Rectal GISTs can occur (3rd most frequent site) and secondarily involve the prostate gland; this phenomenon should be excluded before considering a primary prostatic GIST (7).
Case presentation
A 78-year-old male presented with a history of urinary bladder outflow obstruction symptoms. An ultrasound study demonstrated a markedly enlarged prostate gland, estimated to be ~300 grams [expected ~11 grams (8)]. Non-surgical treatments were attempted to reduce the urinary bladder outflow obstructive symptoms, including microwave treatment and medications (finasteride and doxazosin), without significant improvement. Prostate specific antigen levels were within normal range. Rectal examination revealed a large, non-tender prostate pushing against the anterior rectal wall; no discrete nodules were identified. Despite therapy, he continued to experience increased urinary frequency, urinary retention, nocturia, and constipation. Given these findings, a transurethral resection of the prostate (TURP) gland was performed. The procedure was uneventful, and no suspicious findings were encountered at time of surgery. The preoperative and postoperative diagnoses were presumed to be benign prostatic hyperplasia with urinary retention.
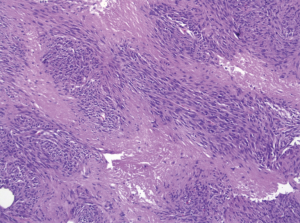
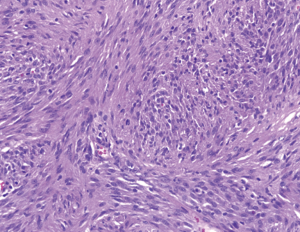
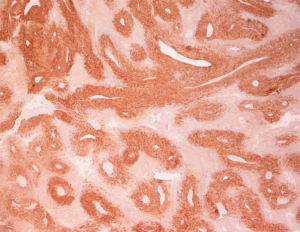
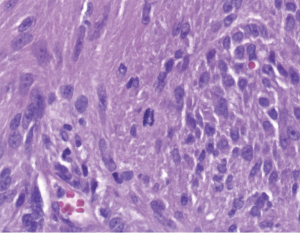
Pathologic examination of the submitted material demonstrated a 36-gram aggregate of gray-pink firm tissue fragments admixed with clotted blood, measuring 11 cm × 8 cm × 2 cm in aggregate. No discrete lesions were noted. Histologic examination of the hematoxylin and eosin stained sections revealed that many of the tissue fragments comprised an abnormal hypercellular proliferation, with areas of hypocellularity imparting a mottled low-power appearance (Figure 1). There were geographic areas of coagulative tumoral necrosis present (Figure 2). High-power examined demonstrated tumor cells with elongate, spindled morphology (Figure 3), with atypical nuclear features including pleomorphism, course granular chromatin, and prominent nucleoli (Figure 4). Mitotic figures were frequently encountered (up to 5 mitoses per 10 high power fields), and atypical mitotic figures were noted (Figure 4). By immunohistochemistry, the neoplastic cells were strongly and diffusely positive for CD117 (Figure 5), vimentin, and CD34. Immunohistochemical stains for smooth muscle actin, desmin, S-100, and cytokeratin cocktail were negative. Given the morphologic and immunohistochemical findings, a pathologic diagnosis of GIST was rendered. The case was sent out for expert consultation; the consulting pathologist agreed with the diagnosis of GIST. Molecular testing for exons 9 and 11 were performed, revealing a KIT exon 11 mutation. There were scattered benign prostatic glandular elements present, some showing associated calcification. No significant atypia was seen in the native prostatic glandular epithelium.

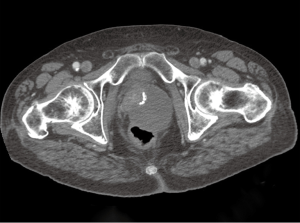
In light of the pathologic findings, computed tomography (CT) scans of the pelvis and abdomen were obtained approximately one month after the surgery. The scan demonstrated a mass in the region of the prostate measuring 10 cm × 9.6 cm. This mass was noted to be contiguous with the anterior rectal wall (Figure 6). There was no regional lymphadenopathy or hepatic lesions identified on the imaging studies. The patient was started on therapy with imatinib mesylate 400 mg once daily, with the possibility of a surgical resection at a later date. Given the patient’s age and potential morbidity, the patient chose not to pursue further surgical treatment. Subsequent imaging studies revealed that the directed therapy with imatinib mesylate had resulted in a significant reduction in the size of the tumor. At twelve months follow-up, there was no evidence of tumor progression or metastatic disease.
Discussion
GISTs are the most frequently encountered primary mesenchymal tumor in the gastrointestinal tract. However, they only account for a small percentage (<2%) of the total gastrointestinal malignancies in the adult population (6). Extra-EGISTs are an exceedingly rare occurrence, accounting for only 5-10% of GISTs (1,3).
Pathologic features that would favor a diagnosis of GIST include cells with spindled and/or epithelioid morphology, perinuclear cytoplasmic vacuolization, and positive immunostaining for CD117, DOG1 and CD34 (5,6). These tumors often show mutations of the KIT or platelet derived growth factor receptor alpha (PDGFRA) genes (9). In addition, BRAF mutations have been reported to occur (9,10). DOG1 immunohistochemical studies may be especially useful, as expression does not appear to be affected by the KIT or PDGFR gene mutation type, and it may be positive in KIT-negative GISTs (9,11).
The pathologic differential diagnosis of spindled neoplasms in the prostate includes schwannoma, melanoma, smooth muscle tumors, solitary fibrous tumor, and prostatic stromal sarcoma. The distinction between GIST and schwannoma can be difficult, as occasional GISTs may show areas suggestive of Verocay bodies. Diffusely and strong immunostaining for S-100 would be typical for schwannoma, while CD117 and smooth muscle markers would be negative. Cytoplasmic clearing and epithelioid cells are typically lacking in schwannoma. While some melanomas may have CD117 positivity, these tumors are DOG1 and CD34 negative, and should stain positively for melanoma tumor markers S100, MART-1, HMB45, and SOX10. Leiomyoma and leiomyosarcoma typically are positive for smooth muscle actin and desmin, and negative for CD117 and CD34. Solitary fibrous tumors are usually CD34 positive, but they should also be BCL-2 positive and CD117 negative. Prostatic stromal sarcoma may be positive for CD34 and progesterone receptor, but has been negative for CD117 in the three reported cases that analyzed this immunostain (5,12,13). A single case has recently been reported implying that prostatic stromal sarcoma may stain positively for DOG1 (12). This suggests that DOG1 positivity should be assessed in combination with other morphologic, immunohistochemical, and molecular studies to achieve the most accurate diagnosis.
In the limited number of primary prostatic GIST described in the literature, the affected patients ranged in age from 31-75 years (mean 51.8 years) (1,2). Other than a single case with liver metastasis, none of the other cases had metastatic disease (1-3). The PSA levels in the cases described have been within normal range (1,2).
Pathologic features used to predict the prognosis of GISTs are tumor size, mitotic rate and location (11). Distinction between GIST and the other entities within the pathologic differential diagnosis is essential, as specific treatment with tyrosine kinase inhibitors is standard of care (5). GISTs and EGISTs do not appear to be successfully treated by typical radiotherapy or chemotherapy, and lymph node metastases are unusual (2,4). Metastatic GISTs may occur in the liver and anyplace in the abdominal cavity, but also on the odd occasion in the lungs or remote peripheral sites (11).
Before diagnosing a primary prostatic GIST, the possibility of a rectal GIST invading and secondarily involving the prostate should be considered (2). Rectal GISTs account for approximately 4% of GISTs, and may be seen as minute intramural nodules ranging to complex pelvic masses with pelvic extension (6,11). They may be connected to the prostate, and may mimic a prostate tumor clinically and on imaging studies (11). GISTs diagnosed at the time of pathologic examination of prostatic specimens appear to more commonly be of rectal than of prostatic origin, and it is somewhat controversial whether primary prostatic GIST is a true entity (7).
In summary, we have described a case of a GIST involving primarily the prostate gland of a 78-year-old man with urinary retention and constipation, discovered at the time of transurethral prostatic resection. The immunoprofile, morphology, and molecular findings are most consistent with a GIST. This lesion was initially thought to represent a primary prostatic GIST, as rectal involvement was not apparent based on initial ultrasound. However, subsequent imaging studies revealed the mass to be contiguous with the anterior rectal wall, suggesting the possibility of a rectal primary with extension to the prostate. GIST should be considered in cases of prostatic tumors with a spindled and/or epithelioid morphology, and immunohistochemistry and possible molecular studies are recommended to aid in diagnosis and guide treatment decisions.
Acknowledgements
Randy Sosolik, MD was involved in original diagnosis of this case.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhang ZH, Feng GW, Liu ZF, et al. A young man with primary prostatic extra-gastrointestinal stromal tumor: a rare case report and review of the literature. Int J Clin Exp Pathol 2014;7:1764-70. [PubMed]
- Liu S, Yu Q, Han W, et al. Primary gastrointestinal stromal tumor of the prostate: A case report and literature review. Oncol Lett 2014;7:1925-9. [PubMed]
- Van der Aa F, Sciot R, Blyweert W, et al. Gastrointestinal stromal tumor of the prostate. Urology 2005;65:388. [PubMed]
- Yinghao S, Bo Y, Xiaofeng G. Extragastrointestinal stromal tumor possibly originating from the prostate. Int J Urol 2007;14:869-71. [PubMed]
- Lee CH, Lin YH, Lin HY, et al. Gastrointestinal stromal tumor of the prostate: a case report and literature review. Hum Pathol 2006;37:1361-5. [PubMed]
- Macías-García L, De la Hoz-Herazo H, Robles-Frías A, et al. Collision tumour involving a rectal gastrointestinal stromal tumour with invasion of the prostate and a prostatic adenocarcinoma. Diagn Pathol 2012;7:150. [PubMed]
- Anagnostou E, Miliaras D, Panagiotakopoulos V. Diagnosis of gastrointestinal stromal tumor (GIST) on transurethral resection of the prostate: a case report and review of the literature. Int J Surg Pathol 2011;19:632-6. [PubMed]
- Leissner KH, Tisell LE. The weight of the dorsal, lateral and medial prostatic lobes in man. Scand J Urol Nephrol 1979;13:223-7. [PubMed]
- Hemminger J, Iwenofu OH. Discovered on gastrointestinal stromal tumours 1 (DOG1) expression in non-gastrointestinal stromal tumour (GIST) neoplasms. Histopathology 2012;61:170-7. [PubMed]
- Hostein I, Faur N, Primois C, et al. BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol 2010;133:141-8. [PubMed]
- Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol 2011;104:865-73. [PubMed]
- Tang YL, Wong CF, Yap WM, et al. Discovered on gastrointestinal stromal tumours 1 (DOG1) expression in non-gastrointestinal stromal tumour (non-GIST) neoplasms. Histopathology 2014;65:724-6. [PubMed]
- Zamparese R, Corini F, Braccischi A, et al. Primary sarcoma of the specialised prostatic stroma: a case report and review of the literature. Case Rep Pathol 2011;2011:252805.