Undifferentiated embryonal sarcoma of the liver with an unusual presentation: case report and review of the literature
Introduction
Undifferentiated embryonal sarcoma of the liver (UESL) is a highly malignant mesenchymal neoplasm of the liver first classified as a distinct clinicopathologic entity in 1978 (1). It predominately occurs in children between 6 and 10 years of age, without gender predilection (1-3), although, it has rarely been reported in adults (4-7). UESL represents about 5-13% of all pediatric hepatic tumors, which are uncommon, and only about 260 cases have been reported in the literature up to 2014. It was reported as a very aggressive neoplasm with a median survival of less than 1 year following diagnosis (1). However, the introduction of the modern supportive therapy and multimodal treatment, including the tumor resection, adjuvant chemotherapy and multiagent chemotherapy, has improved the long-term survival rate and many patients can now be cured (2,6). Therefore, prompt diagnosis and therapy are crucial.
Clinical diagnosis of UESL preoperatively is difficult due to the lack of a characteristic clinical presentation, serological markers and radiological changes. In one report, the diagnosis of UESL was delayed in 23.5% of the cases because of misleading radiologic features (8). Definitive diagnosis of UESL relies on the pathological evaluation of either biopsy or resection specimens. Because the morphological features are relatively nonspecific, and UESL lacks any unique immunohistochemical markers, it is sometimes misdiagnosed as other types of sarcomas involving the liver, including poorly-differentiated or sarcomatoid hepatocellular carcinoma, embryonal rhabdomyosarcoma, and other sarcomas. Therefore, a careful approach is needed for the diagnosis of UESL. Herein, we report our experience on a case of unexpected UESL in a 9-year-old girl with increased IgE, transient eosinophilia and a complex cystic mass in the liver.
Case presentation
A 9-year-old girl with a past medical history of asthma was transferred to our institution with a complaint of right upper quadrant abdominal pain and a recent radiologic evidence of a hepatic mass or cystic lesion. She initially presented to an outside hospital with intermittent right upper quadrant abdominal pain for 1.5 weeks and a palpable abdominal mass. An abdominal ultrasound was read as showing a “Right hepatic complex cyst, suspicious for abscess” and the abdominal computed tomography (CT) scan with contrast demonstrated a 6.4 cm × 5.9 cm, multiloculated, complex hepatic cyst/mass. The initial impression by the CT scan was possible infection such as amoebic or parasitic abscess. The patient was admitted for further evaluation and treatment.
During this admission, a physical examination showed soft, mild tenderness over the liver without organomegaly. Laboratory investigations demonstrated a progressive anemia (Hb, 11.3-9.2 g/dL, normal range: 11.5-15.5 g/dL), increased IgE (152.6 IU/mL, normal range: 0-90.0 IU/mL), transient peripheral eosinophilia (6.8%, normal range: 0-6.5%), increased anion gap (12-19, normal range: 2-11), mildly increased lactate dehydrogenase (253 U/L, normal range: 74-250 U/L) and aspartate aminotransferase (39 U/L, normal range: 0-30 U/L) and increased Westergren sedimentation rate (29 mm/hr, normal range: 0-20 mm/hr). Liver function tests, other liver enzymes, as well as serum alpha-fetoprotein (AFP) were within normal range. No leukocytosis was noted. Furthermore, peripheral blood cultures, stool ova and parasite testing and serological studies for entamoeba and echinococcus were negative. An abdominal ultrasound at our institution again showed a large, round, complex mass in the right lobe of the liver with solid and cystic components and minimal associated vascularity. The imaging findings were felt to favor a predominately avascular process such as a hematoma with an underlying, unidentified process; however an atypical hepatic echinococcal infection or other infection, such as bacterial abscess, was also felt to be possible. Therefore, albendazole therapy for echinococcus was initiated. During this period of time, she did not experience any fever, chills, diarrhea, vomiting or jaundice. However, after 4 weeks of albendazole therapy, a repeat physical examination revealed an enlarged, palpable 8 cm × 5 cm bulging, firm, non tender mass in the right upper quadrant of abdomen below the costal margin. Repeat abdominal CT scan favored a mesenchymal harmatoma of the liver (Figure 1A). Because of the heightened concern for tumor, she underwent surgical resection of the lesion.
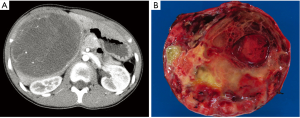
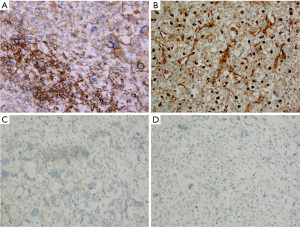
Grossly, the 845 gram, 13.5 cm × 11.5 cm × 9.2 cm resection specimen consisted of a well-encapsulated mass with a small rim of liver parenchyma. The cut surface was soft to gelatinous, variegated, with extensive necrosis and focal hemorrhage (Figure 1B). Minimal cystic degeneration without overt cyst formation was present. Microscopic examination revealed a high grade malignancy with a variable appearance. In some areas the tumor was hypocellular and myxoid, with tumor cells ranging from oval to spindled (Figure 2A). In other areas the tumor was of higher cellularity with marked pleomorphism (Figure 2B). In both patterns of growth, bizarre, multinucleated giant cells with numerous, cytoplasmic hyaline eosinophilic globules were present (Figure 2C). At the periphery, entrapped benign bile ducts were noted, some of which formed elongated collapsed cyst or duct-like structures (Figure 2D). Immunohistochemical staining showed patchy positivity for CD56 (Figure 3A) and weak staining for vimentin (Figure 3B), but were negative for myogenin (Figure 3C), desmin, AFP (Figure 3D) and alpha-antitrypsin. Based on these findings, a diagnosis of UESL was rendered.

Postoperative bone scan, abdominal US, chest, abdominal and pelvis CT did not reveal residual or metastatic disease. A six month course of chemotherapy including ifosfamide and doxorubicin was initiated (Protocol ARST 0332 Treatment ARM C), starting at 2 weeks after the operation, and she tolerated the chemotherapy well. At the time of writing, she is clinically disease-free, 12 months after resection.
Discussion
UESL is a rare and aggressive mesenchymal neoplasm that seems to be unique to the liver, and occurs almost exclusively in children and adolescents. It was first reported as a mesenchymoma (9). Subsequent reports also used other terms, including “mesenchymal sarcoma”, “embryonal sarcoma”, “fibromyxosarcoma”, and “primary sarcoma of the liver. In 1978, Stocker and Ishak distinguished UESL from other sarcomas as a distinct clinicopathologic entity in a report of 31 cases (1).
UESL occurs almost exclusively in children, with a peak incidence between 6 and 10 years of age, without gender predilection. It is very rare after 15 years of age, with only about 70 cases reported, including only 14 cases in patients over 60 years old (4,7). Clinical symptoms of UESL are variable and nonspecific, including abdominal mass with or without abdominal pain, fever, nausea, vomiting, weight loss, fatigue and jaundice. Occasionally, spontaneous rupture may result in intraperitoneal hemorrhage due to rapid growth of the tumor (10-12). Very rare manifestations have included erythropoietin-secreting capacity (13), and life-threatening paraneoplastic syndromes (14). There are no distinctive laboratory markers for this tumor. Mild leukocytosis, low albumin, anemia, elevated lactic dehydrogenase, and normal or mild increased liver enzymes may be seen. Evaluation of some tumor markers including AFP, cancer antigen 19-9 and carcinoembryonic antigen often yield normal results, but AFP may occasionally be elevated (15-18) and one case with increased CA-125 (19) has been reported. Although the clinical and laboratory findings are nonspecific, the combination of presenting symptoms, the age of the patient, and normal AFP levels should raise suspicion of UESL if the clinical context is otherwise appropriate. In the current case, the increased IgE and a transient eosinophilia were initially confusing; although the patient’s history of asthma may explain those findings. The imaging appearance, interpreted as a complex cystic lesion because of the high water content, added to the initial confusion as to the nature of the lesion in our patient. Zaheer et al. reported an adult with UESL who also presented with peripheral eosinophilia and suggested that UESL should be included in the differential diagnosis of eosinophilia accompanying hepatic cysts (15).
Unfortunately, the radiological findings of UESL are also not specific. There are other case reports of UESL that have been mistaken for hydatid disease (15,20-25) or abscess (26,27). In a literature review, Pachera et al. reported that the diagnosis of UESL was delayed in 23.5% of the cases because the presentation of the large cystic mass in the imaging studies was often suggestive of a benign lesion (8). However, these imaging techniques are still helpful to assess the extent of the tumor, any associated invasion of major vessels, biliary obstruction or hilar adenopathy. Typically, ultrasound demonstrates a large mass that may be predominantly solid with many small anechoic spaces. By contrast, CT scan typically demonstrates a hypodense mass with hyperdense septa of variable thickness. Angiographically, UESL is usually hypovascular with tumoral vessels (28,29). Some imaging studies of UESL have demonstrated a large hepatic lesion with a seemingly cystic appearance on CT or MRI images, with a paradoxically solid appearance on ultrasound imaging that was felt to be highly suggestive of UESL (29-33).
Pathological examination of biopsy or surgical resection material, including immunohistochemical analysis, is the mainstay of diagnosis of UESL. Macroscopically, UESL is typically a large, single well-circumscribed mass, often in excess of 10 cm in diameter, and occasionally as large as 35 cm. It is predominately solid, but often has foci of cystic or gelatinous degeneration, hemorrhage, and necrosis. Occasionally, cysts and/or necrosis may occupy up to 80% of the cut surface. Rarely, the tumor may be pedunculated (34). Microscopically, UESL presents as a loose or even myxoid neoplasm of variable cellularity, composed of medium to large spindled, oval or stellate cells with poorly defined cell borders, and intermixed multi-nucleated giant cells, often with severe atypia. Intracellular and extracellular periodic acid-Schiff (PAS)-positive diastase-resistant hyaline globules are often present throughout the neoplasm. In addition, degenerating or dilated biliary duct-like structures surrounded by the neoplastic cells may be present at the periphery, but not deep in the interior of the tumor (34). Extramedullary hematopoiesis is present in about one-half of the cases (35). Immunohistochemically, the staining pattern of UES is variable, and generally not helpful to the diagnosis except as it facilitates the exclusion of other tumors in the differential diagnosis. Vimentin is often positive (34). There is variable staining for glypican-3 (36), CD56, alpha-1 antitrypsin and alpha-1 antichymotrypsin, and paranuclear dot-like staining for cytokeratin has been reported. No immunoreactivity has been described to date for HepPar-1, S-100, GFAP, myoglobin, myogenin, MyoD1, Alk-1, CD34, CD117, smooth muscle myosin heavy chain, h-caldesmon, PE10, or AFP (37-39). A recent study suggested that diffuse membranous expression of CD56 with paranuclear dot-like staining for cytokeratin in the spindled pleomorphic and giant cells of UESL may help in the differential diagnosis of abdominal masses in children and young adults (40).
Currently, there are no universally agreed upon treatment protocols for UESL. Complete resection, combined with adjuvant chemotherapy, appears to be the mainstay of treatment (41,42). Liver transplantation may be an option for patients whose tumors are unresectable or recur, and can result in improved survival (43-45). Historically, the prognosis of UESL was very poor, with 80% 1-year mortality in the report of Stocker and Ishak (1). Another review in 1990 reported a 37.5% disease free survival rate at 3 years (46). Subsequent to the introduction of multimodal therapy, including primary resection, neo-adjuvant or adjuvant chemotherapy, and radiation, the prognosis has improved significantly, and the long-term survival rate in more recent reports ranges from 70% to 100% (2,3,47).
The oncogenesis of UESL remains unclear. No distinctive cytogenetic abnormality has been reported to date. Cytogenetic studies have demonstrated a broad range of complex cytogenetic abnormalities in individual cases of UESL, including gains of chromosome 1q, 5p, 6q, 8p, and 12q, losses of chromosome 9p, 11p and 14 (48), loss of heterozygosity of chromosome 7p, 11p, 17p, 22q, and allelic imbalance of 1p, 8p, 20q (17). There are exceedingly rare cases of UESL arising in or following mesenchymal hamartoma of the liver, in which it harbors the same translocation t(11;19)(q13;q13.4) that can be seen in mesenchymal hamartoma of the liver (49). A few studies have detected point mutations of the TP53 gene in some UESL cases, as well as over-expression of p53 protein in tumoral cells, suggesting the TP53 pathway may be involved in carcinogenesis of UESL, as it is in a number of other tumors (17,50,51). Further investigation of the oncogenesis of UESL is needed, and may eventually facilitate the clinical diagnosis, as well as possibly guiding in the development of better therapies.
In conclusion, we hereby report a case of UESL in a child that was unexpected clinically and radiologically. She was successfully treated with resection and postoperative chemotherapy, and is disease free after relatively short follow-up. Although none of the findings are specific, the diagnosis of UESL is highly suspicious in a young child with a rapidly growing liver mass, especially if the AFP is normal, and the lesion appears cystic on CT imaging but solid on ultrasound study. A definitive diagnosis, however, depends on pathologic examination of either biopsy or resection tissue. Early diagnosis and complete resection are important for a favorable outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer 1978;42:336-48. [PubMed]
- Bisogno G, Pilz T, Perilongo G, et al. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer 2002;94:252-7. [PubMed]
- Ismail H, Dembowska-Bagińska B, Broniszczak D, et al. Treatment of undifferentiated embryonal sarcoma of the liver in children--single center experience. J Pediatr Surg 2013;48:2202-6. [PubMed]
- Lenze F, Birkfellner T, Lenz P, et al. Undifferentiated embryonal sarcoma of the liver in adults. Cancer 2008;112:2274-82. [PubMed]
- Lightfoot N, Nikfarjam M. Embryonal sarcoma of the liver in an adult patient. Case Rep Surg 2012;2012:382723.
- Noguchi K, Yokoo H, Nakanishi K, et al. A long-term survival case of adult undifferentiated embryonal sarcoma of liver. World J Surg Oncol 2012;10:65. [PubMed]
- Hong WJ, Kang YN, Kang KJ. Undifferentiated embryonal sarcoma in adult liver. Korean J Pathol 2014;48:311-4. [PubMed]
- Pachera S, Nishio H, Takahashi Y, et al. Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg 2008;15:536-44. [PubMed]
- Donovan EJ, Santulli TV. Resection of the Left Lobe of the Liver for Mesenchymoma: Report of Case. Ann Surg 1946;124:90-3. [PubMed]
- Uchiyama M, Iwafuchi M, Yagi M, et al. Treatment of ruptured undifferentiated sarcoma of the liver in children: a report of two cases and review of the literature. J Hepatobiliary Pancreat Surg 2001;8:87-91. [PubMed]
- Yu DC, Tandon R, Bohlke AK, et al. Resection of a large, ruptured, undifferentiated (embryonal) sarcoma of the liver in a child: a case report and review of the literature. J La State Med Soc 2009;161:41-4. [PubMed]
- Hung TY, Lu D, Liu MC. Undifferentiated (embryonal) sarcoma of the liver complicated with rupture in a child. J Pediatr Hematol Oncol 2007;29:63-5. [PubMed]
- Lin JM, Heath JE, Twaddell WS, et al. Undifferentiated sarcoma of the liver: a case study of an erythropoietin-secreting tumor. Int J Surg Pathol 2014;22:555-8. [PubMed]
- Fricchione MJ, Glenn N, Follmer R, et al. Life-threatening paraneoplastic syndrome in a child with sarcoma of the liver cured by emergency resection. J Pediatr Hematol Oncol 2013;35:153-5. [PubMed]
- Zaheer W, Allen SL, Ali SZ, et al. Primary multicystic undifferentiated embryonal sarcoma of the liver in an adult presenting with peripheral eosinophilia. Ann Clin Lab Sci 1994;24:495-500. [PubMed]
- Dai CL, Xu F, Shu H, et al. Undifferentiated (embryonal) sarcoma of liver in adult: a case report. World J Gastroenterol 2005;11:926-9. [PubMed]
- Lepreux S, Rebouissou S, Le Bail B, et al. Mutation of TP53 gene is involved in carcinogenesis of hepatic undifferentiated (embryonal) sarcoma of the adult, in contrast with Wnt or telomerase pathways: an immunohistochemical study of three cases with genomic relation in two cases. J Hepatol 2005;42:424-9. [PubMed]
- Sakellaridis T, Panagiotou I, Georgantas T, et al. Undifferentiated embryonal sarcoma of the liver mimicking acute appendicitis. Case report and review of the literature. World J Surg Oncol 2006;4:9. [PubMed]
- Almogy G, Lieberman S, Gips M, et al. Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol 2004;30:421-7. [PubMed]
- Joshi SW, Merchant NH, Jambhekar NA. Primary multilocular cystic undifferentiated (embryonal) sarcoma of the liver in childhood resembling hydatid cyst of the liver. Br J Radiol 1997;70:314-6. [PubMed]
- Charfi S, Ayadi L, Toumi N, et al. Cystic undifferentiated sarcoma of liver in children: a pitfall diagnosis in endemic hydatidosis areas. J Pediatr Surg 2008;43:E1-4. [PubMed]
- Yoon JY, Lee JM. A case of embryonal sarcoma of the liver mimicking a hydatid cyst in an adult. Gut Liver 2010;4:245-9. [PubMed]
- Faraj W, Mukherji D, El Majzoub N, et al. Primary undifferentiated embryonal sarcoma of the liver mistaken for hydatid disease. World J Surg Oncol 2010;8:58. [PubMed]
- Kalra N, Vyas S, Jyoti Das P, et al. Undifferentiated embryonal sarcoma of liver in an adult masquerading as complicated hydatid cyst. Ann Hepatol 2011;10:81-3. [PubMed]
- Oral A, Yigiter M, Demirci E, et al. A case of undifferentiated embryonic liver sarcoma mimicking cystic hydatid disease in an endemic region of the world. J Pediatr Surg 2011;46:e5-9. [PubMed]
- Hanafiah M, Yahya A, Zuhdi Z, et al. A case of an undifferentiated embryonal sarcoma of the liver mimicking a liver abscess. Sultan Qaboos Univ Med J 2014;14:e578-81. [PubMed]
- Xie ZY, Li LP, Wu WJ, et al. Undifferentiated embryonal sarcoma of the liver mistaken for hepatic abscess in an adult. Oncol Lett 2014;8:1184-6. [PubMed]
- Ros PR, Olmsted WW, Dachman AH, et al. Undifferentiated (embryonal) sarcoma of the liver: radiologic-pathologic correlation. Radiology 1986;161:141-5. [PubMed]
- Moon WK, Kim WS, Kim IO, et al. Undifferentiated embryonal sarcoma of the liver: US and CT findings. Pediatr Radiol 1994;24:500-3. [PubMed]
- Buetow PC, Buck JL, Pantongrag-Brown L, et al. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology 1997;203:779-83. [PubMed]
- Lashkari HP, Khan SU, Ali K, et al. Diagnosis of undifferentiated embryonal sarcoma of the liver-importance of combined studies of ultrasound and CT scan. J Pediatr Hematol Oncol 2009;31:797-8. [PubMed]
- Sodhi KS, Bekhitt E, Rickert C. Paradoxical hepatic tumor: Undifferentiated embryonal sarcoma of the liver. Indian J Radiol Imaging 2010;20:69-71. [PubMed]
- Legou F, Ayav A, Cahn V, et al. Radiologic-pathologic comparison of undifferentiated embryonal sarcoma of the liver in a 61-year-old woman. Diagn Interv Imaging 2012;93:e208-11. [PubMed]
- Zimmermann A. eds. Liver tumors of childhood. Practical hepatic pathology: a diagnostic approach 1 Edition. Philadelphia: Saunders, 2011:521-46.
- Goodman ZD, Terracciano LM, Wee A. Tumors and tumor-like lesions of the liver. In: Burt AD, Portmann BC, Ferrell LD, editors. MacSween’s pathology of the liver 6 Edition. Vol. 2. Churchill Livingstone: Elsevier, 2012:761-829.
- Levy M, Trivedi A, Zhang J, et al. Expression of glypican-3 in undifferentiated embryonal sarcoma and mesenchymal hamartoma of the liver. Hum Pathol 2012;43:695-701. [PubMed]
- Keating S, Taylor GP. Undifferentiated (embryonal) sarcoma of the liver: ultrastructural and immunohistochemical similarities with malignant fibrous histiocytoma. Hum Pathol 1985;16:693-9. [PubMed]
- Miettinen M, Kahlos T. Undifferentiated (embryonal) sarcoma of the liver. Epithelial features as shown by immunohistochemical analysis and electron microscopic examination. Cancer 1989;64:2096-103. [PubMed]
- Lack EE, Schloo BL, Azumi N, et al. Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am J Surg Pathol 1991;15:1-16. [PubMed]
- Pérez-Gómez RM, Soria-Céspedes D, de León-Bojorge B, et al. Diffuse membranous immunoreactivity of CD56 and paranuclear dot-like staining pattern of cytokeratins AE1/3, CAM5.2, and OSCAR in undifferentiated (embryonal) sarcoma of the liver. Appl Immunohistochem Mol Morphol 2010;18:195-8. [PubMed]
- Kim DY, Kim KH, Jung SE, et al. Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg 2002;37:1419-23. [PubMed]
- lmogy G, Lieberman S, Gips M, et al. Clinical outcomes of surgical resections for primary liver sarcoma in adults: results from a single centre. Eur J Surg Oncol 2004;30:421-7.
- Dhanasekaran R, Hemming A, Salazar E, et al. Rare case of adult undifferentiated (embryonal) sarcoma of the liver treated with liver transplantation: excellent long-term survival. Case Reports Hepatol 2012;2012:519741.
- Plant AS, Busuttil RW, Rana A, et al. A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): success of combined therapy and the use of orthotopic liver transplant. J Pediatr Hematol Oncol 2013;35:451-5. [PubMed]
- Walther A, Geller J, Coots A, et al. Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl 2014;20:191-9. [PubMed]
- Leuschner I, Schmidt D, Harms D. Undifferentiated sarcoma of the liver in childhood: morphology, flow cytometry, and literature review. Hum Pathol 1990;21:68-76. [PubMed]
- May LT, Wang M, Albano E, et al. Undifferentiated sarcoma of the liver: a single institution experience using a uniform treatment approach. J Pediatr Hematol Oncol 2012;34:e114-6. [PubMed]
- Sowery RD, Jensen C, Morrison KB, et al. Comparative genomic hybridization detects multiple chromosomal amplifications and deletions in undifferentiated embryonal sarcoma of the liver. Cancer Genet Cytogenet 2001;126:128-33. [PubMed]
- Rajaram V, Knezevich S, Bove KE, et al. DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation. Genes Chromosomes Cancer 2007;46:508-13. [PubMed]
- Hu X, Chen H, Jin M, et al. Molecular cytogenetic characterization of undifferentiated embryonal sarcoma of the liver: a case report and literature review. Mol Cytogenet 2012;5:26. [PubMed]
- Sangkhathat S, Kusafuka T, Nara K, et al. Non-random p53 mutations in pediatric undifferentiated (embryonal) sarcoma of the liver. Hepatol Res 2006;35:229-34. [PubMed]


