Pyloric gland adenoma of gallbladder—reports of two cases and a brief review of literature
Introduction
Lesions that protrude from the gallbladder wall into its lumen are called gallbladder polyps, which affect approximately 5% of the adult population and are rarely seen in children (1). Since most affected individuals do not have symptoms from the gallbladder polyps, these are often detected during abdominal ultrasonography performed for other reasons.
Generally, gallbladder polyps can be divided into two classes: benign and malignant. As an uncommon type of benign polyp, gallbladder adenomas are habitually pedunculated single lesions and usually solitary with sizes ranging from 5 to 20 mm. Gallbladder adenomas have four cytological types: pyloric, intestinal, foveolar and biliary (2).
It has been well established that adenomas and dysplasia are the precursor lesions of colorectal adenocarcinoma (3). However, whether there is direct adenoma-carcinoma sequence in the gallbladder is still controversial. Some researchers consider adenoma a premalignant lesion after observing malignant change on follow-up of adenomas, and finding adenomatous residue in 19% of invasive carcinomas (4). However, others believe adenoma only plays a minor role in gallbladder carcinogenesis (5).
The prognosis of gallbladder cancers is extremely poor with 5-year survival in early stage (T2) at 29%. Early diagnosis is difficult due to a lack of specific signs or symptoms. Therefore, it is important to differentiate between benign polyps and malignant or premalignant polyps (1).
Here we present two cases of gallbladder adenomas, their histopathologic features and possible role in malignant transformation, along with a brief literature review.
Case presentation
Case 1
A 40-year-old Hispanic male presented to hospital with an enlarging gallbladder polyp, which was noted originally to be 5 mm in June of the previous year and then enlarged to 9 mm in March of the following year. The patient reported abdominal pain, but denied it being related to the timing or type of food.
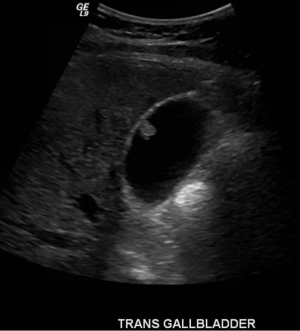
Thirteen years earlier this patient had rapid 70 pound weight gain and asymptomatic abnormal liver enzymes. He was diagnosed with fatty liver and was advised weight loss. Five years earlier, he presented with right upper quadrant pain, accompanying by elevated liver enzymes, positive antinuclear antibody (ANA), and smooth muscle antibody. Subsequent endoscopic retrograde cholangiopancreatography (ERCP) showed mild stricture of the common bile duct without stones. A liver biopsy showed granulomas, minimal fatty change, and mild piecemeal necrosis. Following abdominal ultrasound, three years later, a 5 mm polyp was discovered in the gallbladder, with a slightly prominent common bile duct. This polyp progressed to 8-9 mm in diameter within 2 years (Figure 1), and the common bile duct remained unchanged.
A subsequent Robotic assisted single port cholecystectomy and liver biopsy were performed and during operation, minimal adhesions were noted to the gallbladder.
The gross exam revealed a 33 gram, 6.4 cm × 3.6 cm × 2.8 cm gallbladder, with 1.2 cm × 0.7 cm × 0.4 cm polyp and occasional polypoid mucosal projections ranging from 0.1 to 0.5 cm in the lumen. The remaining mucosal surface was tan with yellow striations.
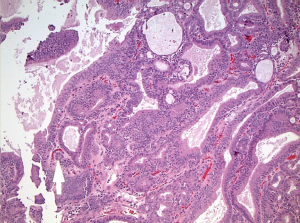
Histopathological examination after routine processing and paraffin-embedding revealed a polyp of the gallbladder with extensive tubuloglandular and focal cribriform features (Figure 2). Foveolar or intestinal type metaplasia was not a prominent feature. Cytologically, the cells appeared benign with cuboidal to columnar appearances and round to oval nuclei (Figure 3). The focal cribriform areas were of concern for low grade malignancy, but no increase in mitotic figures or features to support malignancy were noted. Also present were several “squamoid morules” which consisted of vague spindle cell whorls without keratinization (Figure 4). The background mucosa showed cholesterolosis.

Immunohistochemical studies showed positive caudal-related homeobox transcription factor 2 (CDX2) expression in most parts and brisk Ki-67 labeling indices.
Liver biopsy showed mild to focally moderate portal lymphoplasmacytic inflammation, mild steatosis, mild portal fibrosis with focal sinusoidal extension, and patchy minimal increase in iron stores.
Case 2
A 53-year-old non-Hispanic male presented to hospital for evaluation of his gallbladder polyp and symptomatic cholelithiasis. This polyp had not changed significantly for 2 years since it was detected. Patient denied abdominal pain, jaundice, significant weight loss, fatigue, changes in urine or stool color, nausea, and vomiting. He was managing his cholelithiasis by moderating his diet. Two years prior, this patient was diagnosed with diffuse large B-cell lymphoma, stage 4, and was in remission after six cycles of R-CHOP [rituximab + cyclophosphamide + hydroxydaunomycin (doxorubicin hydrochloride) + oncovin (vincristine sulfate) + prednisone/prednisolone] chemotherapy.
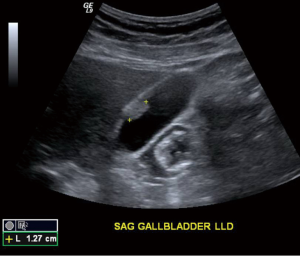
Abdominal ultrasound re-demonstrated a gallbladder polyp along the nondependent wall measuring 1.3 cm in maximal transverse diameter, (Figure 5), as well as cholelithiasis without evidence of cholecystitis. A laparoscopic cholecystectomy was performed subsequently.
The gross exam revealed a 13 gram, 8.4 cm × 2.4 cm × 1.2 cm gallbladder, with a 1.6 cm × 1.0 cm × 0.4 cm detached polyp in the lumen. Within the fundic portion of the gallbladder was a 1.2 cm × 1.2 cm cystic structure. The remaining mucosa was tan with occasional yellow speckling.
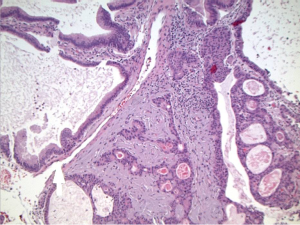
Histopathologic sections showed a polyp made up of compact, mostly tubular glands with a hint of papillary change in rare foci (Figure 6). The glands were made of low cuboidal or columnar epithelium, with mostly gastric type differentiation and some biliary overlay (Figure 6), but lacking in obvious goblet cell/classic intestinal features; the glands were closely packed with virtually no intervening stroma in most parts. No squamoid morules were seen, but some glands contained bile. There was mild focal dysplasia in a few deeper glands (Figure 7), but no evidence to suggest that this was a premalignant entity.
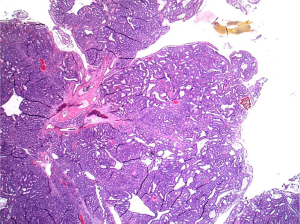
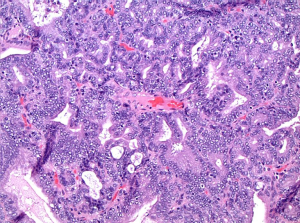
On immunohistochemical studies, the epithelium was cytokeratin 7 (CK7) and CDX2 positive, with rare synaptophysin-immunoreactive cells and somewhat brisk Ki-67 labeling indices. Mitoses were inconspicuous.
Discussion
Gallbladder polyps are found in approximately 5% of the worldwide population (1) and its diagnosis has increased greatly due to widespread use of abdominal ultrasound. Gallbladder polyps are mostly seen in adults with age ranging from 20 to 94 years (6). They are classified as benign or malignant. Benign gallbladder polyps are subdivided into: pseudotumors (cholesterol polyps, inflammatory polyps; cholesterolosis and hyperplasia), epithelial tumors (adenomas) and mesenchymatous tumors (fibroma, lipoma, and hemangioma). Amongst these cholesterol types are the most common benign polyps (78.5%); whereas adenomas only account for 2.2% (7). Malignant polyps present as gallbladder carcinomas.
Adenomas, which account for 10% of ultrasonographically diagnosed gallbladder polyps (6), have been reported in 0.3% to 0.5% of gallbladders removed for either cholelithasis or chronic cholecystitis. They are usually asymptomatic except when adenomas are multiple, large, or detached, which results in free-floating fragments within the bile ducts or associated with gallstones causing symptomatic cholelithiasis. In contrast, adenomas of the extrahepatic bile ducts typically present with signs and symptoms of obstruction (8). Patients with gallbladder adenomas often have gallstones, chronic cholecystitis, and pyloric gland metaplasia. Rarely, adenomas are detected in association with biliary papillomatosis, familial adenomatous polyposis coli/Gardner’s syndrome, Peutz-Jeghers syndrome, or in patients with an anomalous union of the pancreatobiliary duct (9,10).
The main growth patterns of gallbladder adenomas are similar to those in colonic adenomas: tubular, papillary (villous), and tubulopapillary (tubulovillous). The tubular type is most prevalent and is composed of pyloric or intestinal type glands. The tubulopapillary pattern should have at least 20% of both tubular and papillary patterns. Apparently, a prominent papillary component is related to increased chances of malignant transformation. The frequencies of growth patterns in gallbladder adenomas are shown in Table 1.

Full table
Cytoarchitecturally, pyloric, intestinal, foveolar, and biliary are the four types of gallbladder adenomas and the frequencies of each type is shown in Table 2 (12). Despite of these classifications, gallbladder adenomas often have heterogeneous cell populations and display a wide range of morphological overlap, which makes histologic interpretation difficult (2).
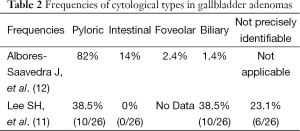
Full table
Pyloric gland adenoma is the most common histological type of gallbladder adenoma, which has epithelium morphologically similar to gastric pyloric glands or duodenal Brunner’s glands (8). Usually pyloric type has tightly packed, small, round, and relatively uniform glands, with or without intervening stroma. Sometimes areas of Paneth cells or endocrine cells with some mucinous or squamous differentiation can be identified (13).
“Squamous morules” (squamous spindle cell metaplasia), the vague whorls of epithelial cells without keratinization (immature), can be seen in 24.1-35% of pyloric gallbladder adenomas (12,13). The squamous morules consist of oval or spindle-shaped, bland, uniform appearing cells lacking prominent nucleoli, Moreover, sometimes biotin-rich optically clear nuclei can be identified in these cells (12). It is worth mentioning that such “squamous morules” are not seen in other types of gallbladder adenomas and therefore can be considered at a characteristic histologic marker of pyloric gland adenoma (12).
As the most common malignancy of the biliary tree and the fifth most common gastrointestinal cancer, gallbladder cancer is diagnosed in 6,000 patients every year in the United States (14). Because gallbladder cancer does not have specific signs or symptoms on presentation, its prognosis is very poor and it is most often discovered incidentally after cholecystectomy (15). Microscopically, most gallbladder cancers are adenocarcinomas showing varying degrees of differentiation (16).
Some researchers believe adenoma may play a role in some cases of gallbladder cancer. Based on the observation of 7 adenomas with malignant change and evidence of adenomatous residue in 15 of 79 (19%) invasive carcinomas in a study of 1,605 resected gallbladder specimens, Kozuka et al. suggested an adenoma-carcinoma sequence (4). In the study of 1,847 cholecystectomized specimens from a Korean population, Lee et al. found that malignant transformation occurred in 23.5% of gallbladder adenomas (11). In addition, as in the colorectum, the larger the adenoma is, the more likely that an area of malignant change will be found.
However, Yanagisawa et al. noticed major differences in beta-Catenin exon 3 mutations between adenomas and carcinoma of gallbladder (62.5% vs. 4.8%) (5); similarly, TP53, KRAS and p16 mutations are frequently found in gallbladder carcinomas but not in gallbladder adenomas (17). This evidence suggests that the adenoma-carcinoma sequence is only a minor pathway of gallbladder carcinogenesis.
Many immunohistochemical markers have been employed in the differentiation of gallbladder adenomas and malignant forms; the most common markers are mucin markers, including mucin 2 (MUC2), MUC5AC, MUC6, human gastric mucin (HGM) and mucin characteristic of gastric gland mucous cells (M-GGMC-1), CDX2, cluster of differentiation 10 (CD10), and sometimes MUC1, CK7 and CK20 (12,13,18-20). Table 3 summarizes these markers and their expression in different types of gallbladder adenomas and gallbladder carcinomas.
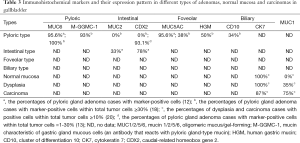
Full table
CDX2 is a member of the caudal type homeobox gene family; with the encoded protein playing an important role in early embryonic development of the intestinal tract, intestinal inflammation and tumorigenesis (21). Mostly CDX2 expression is identified in the villi or differentiated cells of the intestine and therefore CDX2 is considered as an intestinal marker in gallbladder adenoma (18). However, Wani et al. observed frequent CDX2 positivity in 93.1% pyloric type adenoma, and concluded that CDX2 expression is not only related to intestinal metaplasia of goblet, Paneth and endocrine cells in pyloric type adenoma, but was also associated with squamous morules and stable beta-catenin. So they proposed that CDX2 expression is just one characteristic of pyloric type adenomas of gallbladder (13).
Based on the features of case 1, especially the presence of “squamoid” morules within the stroma and the profile of Immunohistochemical markers, a diagnosis of ‘pyloric’ gland type of adenoma was made.
Case 2 shared some similarities with case 1 on histopathological and immunohistochemical profiling, and although not typical, was reminiscent of a ‘pyloric’ gland type of adenoma. However, it had some biliary features and “squamoid” morules were not observed. The nomenclature on such low-grade polypoid gall bladder entities is changing. Current terminology [based on Dr. Odze’s discussion published in September, 2014, (22)] this polyp is best regarded an intracholecystic papillary-tubular neoplasm (ICPN), and subtyped as a pyloric type adenoma (22). ICPNs include different discrete, mass-forming, preinvasive neoplasms that arise in gallbladder mucosa and may be considered the gallbladder counterparts of gastrointestinal adenomas and pancreatic and biliary intraductal neoplasms (22).
The risk of malignancy is high in patients over 50 years old who have single polyps with diameters >10 mm. Surgical resection of gallbladder polyps is recommended in patients with symptoms such as biliary-type pain and dyspepsia. For asymptomatic individuals, resection is suggested (1) if (I) they are over 50 years old; (II) their polyps are solitary and greater than 10 mm in size; (III) they have concurrent gallstones, or IV) their polyps grow continuously on ultrasound exams. For polyps 6-9 mm in diameter with no signs of malignancy, a repeat ultrasound in 6 months is recommended. If there are no significant a change, a new ultrasound in 12 months is advised; and if these exams are both stable, no further imaging studies are needed (2).
Acknowledgements
The authors thank Mary Dennis, Andrea Hartwick and Maria Rabina (MHS PA) for assistance in specimen grossing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Myers RP, Shaffer EA, Beck PL. Gallbladder polyps: epidemiology, natural history and management. Can J Gastroenterol 2002;16:187-94. [PubMed]
- Andrén-Sandberg A. Diagnosis and management of gallbladder polyps. N Am J Med Sci 2012;4:203-11. [PubMed]
- Fleming M, Ravula S, Tatishchev SF, et al. Colorectal carcinoma: Pathologic aspects. J Gastrointest Oncol 2012;3:153-73. [PubMed]
- Kozuka S, Tsubone N, Yasui A, et al. Relation of adenoma to carcinoma in the gallbladder. Cancer 1982;50:2226-34. [PubMed]
- Yanagisawa N, Mikami T, Saegusa M, et al. More frequent beta-catenin exon 3 mutations in gallbladder adenomas than in carcinomas indicate different lineages. Cancer Res 2001;61:19-22. [PubMed]
- Ito H, Hann LE, D’Angelica M, et al. Polypoid lesions of the gallbladder: diagnosis and followup. J Am Coll Surg 2009;208:570-5. [PubMed]
- Matos AS, Baptista HN, Pinheiro C, et al. Gallbladder polyps: how should they be treated and when?. Rev Assoc Med Bras 2010;56:318-21. [PubMed]
- Odze RD, Goldblum JR, editors. Surgical pathology of the GI tract, liver, biliary tract, and pancreas (2e). Elsevier Health Sciences, 2009:846-51.
- Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press, 2000:203-10.
- Wada K, Tanaka M, Yamaguchi K, et al. Carcinoma and polyps of the gallbladder associated with Peutz-Jeghers syndrome. Dig Dis Sci 1987;32:943-6. [PubMed]
- Lee SH, Lee DS, You IY, et al. Histopathologic analysis of adenoma and adenoma-related lesions of the gallbladder. Korean J Gastroenterol 2010;55:119-26. [PubMed]
- Albores-Saavedra J, Chablé-Montero F, González-Romo MA, et al. Adenomas of the gallbladder. Morphologic features, expression of gastric and intestinal mucins, and incidence of high-grade dysplasia/carcinoma in situ and invasive carcinoma. Hum Pathol 2012;43:1506-13. [PubMed]
- Wani Y, Notohara K, Fujisawa M. Aberrant expression of an “intestinal marker” Cdx2 in pyloric gland adenoma of the gallbladder. Virchows Arch 2008;453:521-7. [PubMed]
- Herman JM, Pawlik TM, Thomas CR Jr. Biliary tract and gallbladder cancer: a multidisciplinary approach. Springer SBM 2014:2-5.
- Boutros C, Gary M, Baldwin K, et al. Gallbladder cancer: past, present and an uncertain future. Surg Oncol 2012;21:e183-91. [PubMed]
- Feldman AL, Yi ES. Rosai and Ackerman’s surgical pathology. JAMA 2012;307:201.
- Kim YT, Kim J, Jang YH, et al. Genetic alterations in gallbladder adenoma, dysplasia and carcinoma. Cancer Lett 2001;169:59-68. [PubMed]
- Nagata S, Ajioka Y, Nishikura K, et al. Co-expression of gastric and biliary phenotype in pyloric-gland type adenoma of the gallbladder: immunohistochemical analysis of mucin profile and CD10. Oncol Rep 2007;17:721-9. [PubMed]
- Kalekou H, Miliaras D. Cytokeratin 7 and 20 expression in gallbladder carcinoma. Pol J Pathol 2011;62:25-30. [PubMed]
- Chang HJ, Kim SW, Lee BL, et al. Phenotypic alterations of mucins and cytokeratins during gallbladder carcinogenesis. Pathol Int 2004;54:576-84. [PubMed]
- Yan LH, Wei WY, Xie YB, et al. New insights into the functions and localization of the homeotic gene CDX2 in gastric cancer. World J Gastroenterol 2014;20:3960-6. [PubMed]
- Odze RD, Goldblum JR, editors. Surgical pathology of the GI tract, liver, biliary tract, and pancreas (3e). Elsevier Health Sciences 2014:1022-25.