
The association between obesity factor and esophageal caner
Introduction
The incidence of esophageal adenocarcinoma (EA) is rapidly increasing in many Western countries, carrying a poor prognosis and a strong male predominance. The disease has increased as high as 5-fold in the United States over the past three decades according to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry data (1). There is still no consensus regarding the cause of the rise in EA incidence, though increasing gastroesophageal reflux disease (GERD), use of nonsteroidal anti-inflammatory drugs, eradication of Helicobacter pylori infection (2,3), and obesity have all been suggested (4). Among these risk factors, obesity has received particular attention as a potential causal factor in the rapid rise in incidence of EA (4).
The increasing occurrence of EA might be explained by the increasing weight trends in Western society, but a careful review of the existing data is required before such conclusions can be drawn. Over the past two decades, there have been an increasing number of well designed epidemiological studies which have furthered understanding of the influence of obesity on the development of EA. Two meta-analyses have shown the risk of EA in overweight and obese individuals increased approximately 2- to 3-fold (5,6) and is higher in obese individuals than in those who are simply overweight (7), consistent with an exposure-response effect. Furthermore, obesity has been associated with symptoms of GERD and Barrett’s esophagus (7-9). These findings, coupled with the high temporal correlation between obesity prevalence and EA incidence, have led to speculation that the “obesity epidemic’’ in the United States may be at least partially responsible for the increase in EA incidence (10,11).
This review provides an update on the role of obesity in the risk of developing esophageal adenocarcinoma. The correlation of obesity and esophageal adenocarcinoma as well as the potential mechanisms underlying these effects are also discussed.
Obesity in general
Two surveys from US National Health and Nutrition Examination Survey show the prevalence of obesity increased from 15% in 1976-1980 to 33.8% in 2007-2008 (12). Sixty-eight percent of US adults aged 20 years or older are overweight or obese, and 34% are obese currently (13). In Europe, it is estimated that 30-80% adults are currently overweight, and the World Health Organization (WHO) predicts the prevalence of obesity is expected to include 150 million adults and 15 million children by 2010 (14). In the Asian-Pacific area, Australia has the highest prevalence of overweight (31%) and obese adults (20%). In China the prevalence of overweight and obese patients has increased by almost four fold during the last two decades.
Measure of obesity
Several measures are used to assess obesity. Body mass index (BMI), an individual’s body weight (in kg) divided by the square of their height (in m), is an easily available measure often used for obesity assessment in epidemic studies. The WHO divides BMI into four categories: a BMI <18.5 represents an underweight state; a BMI between 18.5-24.9 represents normal weight; a BMI between 25.0-29.9 represents an overweight state, and a BMI ≥30 represents obesity. Other anthropometric measurements of obesity include waist circumference and waist:hip ratio, which better reflect the differential distribution of body fat than BMI alone. Use of radiologic techniques such as computerized tomography (CT) also allows the level of intra-abdominal fat to be assessed. Sophisticated technologies like dualenergy X-ray absorptiometry can provide an overall and regional assessment of body composition.
Esophageal cancer in general
Esophageal cancer represents the eighth most common cancer worldwide and the sixth most common cause of cancer death, with 462,000 new cases in 2002 (4.2% of the total) alone (15,16). The case fatality ratio is as high as 83%, much higher than that of other common malignancies like breast cancer (36%) or colorectal cancer (52%) (16). It is estimated that the incidence of the disease has increased more than five-fold in some countries over the last three decades (17). In worldwide, there are an estimated 482,300 new esophageal cancer cases and 406,800 deaths occurred in 2008 (18). In the US, an estimated 17,460 cases of esophageal cancer will be diagnosed in 2012, with 15,070 deaths expected from the disease (19).
Adenocarcinoma now accounts for around 50% of all esophageal cancers in the West, while the incidence of squamous cell carcinomas (SCC), previously the most common histology in the West, has been decreasing by 3.6% per year from 1998 to 2002 across all ethnic groups in the US (19). The incidence of distal and junctional EA rose approximately six-fold from 4 cases per million in 1975 to 23 cases per million in 2001, strongly indicating a true increase in disease burden not explained by over-diagnosis or reclassification (17).
Esophageal adenocarcinoma and obesity
Obesity is reported to be associated with an increased risk of many malignancies, including tumors of the colon, kidney, liver, thyroid, gall bladder, pancreas and endometrium (20,21). The earliest reports about a possible association between obesity and esophageal adenocarcinoma were published in the mid 1990s (22,23). These findings were corroborated in large populationbased, case-control studies conducted in the US, Europe and Australia, which showed a strong correlation between increasing BMI and the risk of developing EA (4,24) and further supported by the findings of prospective cohort studies (Table 1).
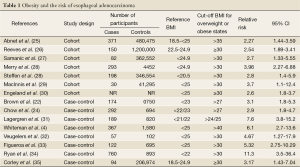
Full table
In an 8 year follow up period, 371 cases of EA were identified in 480,475 participants in the National Institute for Health AARP Diet and Health study cohort and those with a BMI in the highest category (>35) were at increased risk (Relative risk, RR, 2.27; 95% Confidence interval, 95% CI: 1.44-3.59) (25). A prospective cohort study of 1.2 million women (50-64 years) during 1996-2001 showed that individuals with a BMI >30 were at increased risk (26). Samanicet al. examined the health records of 362,552 men for an average of 19 years and showed that there’s a significantly increased risk of EA in Obese (BMI >30) compared to normal weight men (27).
In another study of 4552 subjects over 13.3 years, Merry et al. found the RR of EA to be 1.4 (95% CI: 0.95-2.04, P<0.001)for overweight subjects and 3.96 (95% CI: 2.27-6.88, P<0.001) for obese subjects, respectively (28). In the European Prospective Investigation into Cancer and Nutrition, 346,544 adults were followed for 8.9 years. BMI, waist-hip ratio and waist circumference were all positively associated with EA (RR 2.60, 95% CI: 1.23-5.51, P<0.01; RR, 2.12; 95% CI: 0.98-4.57, P<0.004 and RR, 3.07; 95% CI: 1.35-6.98, P<0.003, respectively) (36). The MacInnis group followed 41,295 subjects over 11 years, with detailed body composition information from bioelectrical impedance analysis performed at baseline. They found the hazard ratio (HR) of adenocarcinoma of the lower esophagus for individuals with a BMI >30 versus a BMI <25 was 3.7 (95% CI: 1.1-12.4, P<0.03). What’s more, for every 10 cm increase in waist circumference the HR was 1.46 (95% CI: 1.0-2.04) and for every 10 kg increase in fat free mass the HR was 2.06 (95% CI: 1.15-3.69) (36). Another prospective study which measured the height and weight of approximately 2 million Norwegians showed that, compared with persons of normal weight (BMI 18.5-24.9), men and women who were overweight (BMI 25.0-29.9) had a relative risk (RR) of 1.8 (95% CI 1.48-2.19) and 1.6 (95% CI 1.08-2.49) of developing esophageal adenocarcinoma, respectively. The corresponding relative risks in men and women who were obese (BMI ≥30) were 2.6 (95% CI 1.8-3.7) and 2.1 (95% CI 1.3-3.4), respectively (30).
In a population-based case-control study, Whiteman’s group compared 367 cases of EA, 426 cases of Gastroesophageal junction adenocarcinoma and 1,580 controls. Morbidly obese individuals (BMI >40) had a significantly increased risk of EA with odds ratio (OR) 6.1 (95% CI: 2.7-13.6, P<0.001). The authors reported the risk was significantly higher for males than females, and for obese people with reflux (OR 16.5, 95% CI: 8.9-30.6) than obese people without reflux (OR 2.2, 95% CI: 1.1-4.3), suggesting a synergistic interaction between these factors (4). Ryan and colleagues conducted a case-control study of 508 EA cases and 893 controls (34). A dose-dependent relationship between pre-illness BMI and EA was observed for males (OR 4.3, 95% CI 2.3-7.9, P<0.0001) in the highest BMI quartile versus the lowest. Especially for the lower esophagus, an OR of 11.3 (95% CI 3.5-36.4, P<0.001) was observed and for the gastroesophageal junction the OR was 3.4 (95% CI: 1.4-8.7, P<0.001).
Mechanism
It was proposed that an increased occurrence of GERD among individuals who are obese can lead to occurrence of Barrett’s esophagus and finally esophageal adenocarcinoma, a likely mechanism explaining the association between abdominal adiposity and esophageal adenocarcinoma (9). However, the associations between BMI or adiposity and this tumor were seemingly independent of the symptoms of GERD in virtually all studies with GERD data (4,24,31,35,37). These results indicate that obesity might have an independent carcinogenic role in occurrence of esophageal adenocarcinoma. Nevertheless, since the mechanisms underlying the association between obesity and esophageal adenocarcinoma are not fully established, further research into the potential role of GERD in the carcinogenic pathway is warranted.
There are several molecular mechanisms that can contribute to the increased risk of cancer among obese individuals (21), but the reasons behind the robust and specific association between obesity and esophageal adenocarcinoma still remain to be clarified. Research into the mechanisms underlying this association, however, is emerging.
The insulinlike growth factor (IGF) pathway, which is associated with obesity, plays an important role in regulating cell proliferation, differentiation, apoptosis, and transformation. Laboratory studies have shown that IGFs exert strong mitogenic and antiapoptotic actions on various cancer cells. The IGF pathway has been shown to be associated with increased risk for several common cancers including breast (38), prostate (39), lung (40) and colorectum (41), and may represent a mechanistic link between obesity and esophageal adenocarcinoma. Recent studies have shown that polymorphisms in genes encoding proteins belonging to the IGF family could be markers of increased risk of esophageal adenocarcinoma (36). In a case-control study of 431 wellcharacterized individuals in Canada, Kimberley Macdonald et al. analyzed the frequency of the 1013G>A polymorphism in the gene encoding the IGFI receptor in a series and showed that individuals who were obese and carried the 1013G>A variant had a higher risk of developing esophageal adenocarcinoma than those who carried the 1013G>G variant. Thus, this commonly occurring gene polymorphism might modulate the risk of esophageal adenocarcinoma in individuals who are obese, probably by altering the function of the IGFI receptor (42).
The cytokines leptin and adiponectinsecrected by adipocytes are other factors that might contribute to the link between obesity and esophageal adenocarcinoma. Serum leptin levels rise with an increase in BMI (43). It has been shown that leptin stimulates proliferation and inhibits apoptosis in esophageal adenocarcinoma cells (43). In addition, this hormone can activate the epidermal growth factor receptor, an important signaling mechanism for activation of Gproteincoupled receptors, and promote cell proliferation (43). Adiponectin is the most abundant protein secreted by adipose tissue and is known to be involved in various obesityrelated disorders (44). The serum concentrations of adiponectin, unlike most of the other adipokines, are inversely correlated with BMI and most importantly, with visceral fat accumulation (45). A study of 75 patients with esophageal adenocarcinoma indicated that obesity was associated with up-regulated expression of the leptin receptor and the two adiponectin receptors in tumor specimens from these patients. The increase in the expression of two of these receptors (LEPR and ADIPOR2) was associated with advanced tumor stages, suggesting that pathways involving adipokines affect tumor biology (46).
In 1998, Lagergrenet al. (47) hypothesized that sex hormones could be responsible for the sex imbalance occurrence of esophageal carcinoma. Epidemiological data for esophageal adenocarcinoma demonstrates a profound gender difference, with the male to female ratio exceeding 8:1, strongly supporting this hypothesis (48-50). Estrogen has also been shown to contribute to the regulation of body adiposity and fat distribution through ERs in the brain, decreasing insulin sensitivity and increasing leptin signaling pathways (51). 17β-estradiol increases leptin mRNA levels in adipose tissue (52), while estrogen deficiency impairs central leptin sensitivity (51,53). In women, fluctuations of leptin during the menstrual cycle correlate directly with levels of estrogen (52,54). Estrogen has also been found to influence leptin receptor expression and sensitivity of hypothalamus to leptin, driving subcutaneous body fat accrual over visceral fat during the estrous cycle in rats (55). Hence, visceral fat varies inversely with estrogen levels as seen visceral fat accumulate in postmenopausal women with sufficiently low circulating estrogen levels (46,53,56). The accumulation of visceral fat is associated with an increased risk of various gastrointestinal malignancies including esophageal adenocarcinoma (47). Thus, estrogen regulation of leptin levels in women may play a protective role, directing accumulation of subcutaneous fat preferentially over visceral fat. The situation for men, however, is less clear, although a high level of leptin is considered to be a risk factor for males to develop esophageal adenocarcinoma (8,47).
Conclusions
Large epidemiological studies have highlighted a marked increase in esophageal adenocarcinoma over the last 30 years, making this histologic subtype the most common esophageal cancer in the West (15). The factors underlying the increased incidence of EA are complicated. The link between increased BMI, central obesity and this cancer is supported by a large body of evidence. It is likely that the obesity epidemic explains, at least in part, the increasing incidence of esophageal adenocarcinoma observed in Western countries over the past few decades (13). Moreover, the male predominance of this tumor might be partly explained by its strong association with body fat distribution typical to men.
It is becoming increasingly apparent that insulin resistance, disturbed adipokine homeostasis secondary to central adiposity, and sex hormones all lead to activation of carcinogenic molecular pathways and may explain the gender and ethnic differences seen in this cancer. There are still uncertainties regarding the role of obesity in the increased incidence of esophageal adenocarcinoma. Much research remains to be carried out before the mechanisms that explain the strong link between obesity and esophageal adenocarcinoma are fully understood. The interactions between obesity and other environmental exposures including tobacco smoking, infection with Helicobacter pylori and dietary factors also deserve attention (9). Dietary and lifestyle modification aimed at avoidance of central obesity will likely provide the most benefit in the prevention of esophageal and other cancers.
Acknowledgements
We appreciate Dr. Qi Cao and Dr. Jun Wang for the critical reading. The work was supported by National Natural Science Foundation of China (Grant No. 81172244).
Disclosure: The authors declare no conflict of interest.
References
- Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001;92:549-55. [PubMed]
- Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila) 2008;1:329-38. [PubMed]
- Vaughan TL, Kristal AR, Blount PL, et al. Nonsteroidal anti-inflammatory drug use, body mass index, and anthropometry in relation to genetic and flow cytometric abnormalities in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 2002;11:745-52. [PubMed]
- Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut 2008;57:173-80. [PubMed]
- Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med 2005;143:199-211. [PubMed]
- Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:872-8. [PubMed]
- Jacobson BC, Somers SC, Fuchs CS, et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006;354:2340-8. [PubMed]
- Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett’s esophagus. Gastroenterology 2007;133:403-11. [PubMed]
- Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol 2011;8:340-7. [PubMed]
- Jeon J, Luebeck EG, Moolgavkar SH. Age effects and temporal trends in adenocarcinoma of the esophagus and gastric cardia (United States). Cancer Causes Control 2006;17:971-81. [PubMed]
- Kort EJ, Sevensma E, Fitzgerald TL. Trends in esophageal cancer and body mass index by race and gender in the state of Michigan. BMC Gastroenterol 2009;9:47. [PubMed]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii,1-253. [PubMed]
- Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235-41. [PubMed]
- Branca F, Nikogosian H, Lobstein T, et al. Regional Office for Europe. The challenge of obesity in the WHO European region and the strategies for response: summary. Copenhagen: World Health Organization, Regional Office for Europe, 2007.
- Ryan AM, Duong M, Healy L, et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: epidemiology, etiology and new targets. Cancer Epidemiol 2011;35:309-19. [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005;97:142-6. [PubMed]
- Nattermann C, Dancygier H. Endoscopic sonography in esophageal cancer. Leber Magen Darm 1992;22:177-83. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [PubMed]
- Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst 1995;87:104-9. [PubMed]
- Vaughan TL, Davis S, Kristal A, et al. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 1995;4:85-92. [PubMed]
- Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1998;90:150-5. [PubMed]
- Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465-71. [PubMed]
- Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335:1134. [PubMed]
- Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006;17:901-9. [PubMed]
- Merry AH, Schouten LJ, Goldbohm RA, et al. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut 2007;56:1503-11. [PubMed]
- MacInnis RJ, English DR, Hopper JL, et al. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer 2006;118:2628-31. [PubMed]
- Engeland A, Tretli S, Bjørge T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control 2004;15:837-43. [PubMed]
- Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999;130:883-90. [PubMed]
- Veugelers PJ, Porter GA, Guernsey DL, et al. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus 2006;19:321-8. [PubMed]
- Figueroa JD, Terry MB, Gammon MD, et al. Cigarette smoking, body mass index, gastro-esophageal reflux disease, and non-steroidal anti-inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control 2009;20:361-8. [PubMed]
- Ryan AM, Rowley SP, Fitzgerald AP, et al. Adenocarcinoma of the oesophagus and gastric cardia: male preponderance in association with obesity. Eur J Cancer 2006;42:1151-8. [PubMed]
- Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev 2008;17:352-8. [PubMed]
- McElholm AR, McKnight AJ, Patterson CC, et al. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology 2010;139:204-12.e3.
- Lindblad M, Rodríguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control 2005;16:285-94. [PubMed]
- Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998;351:1393-6. [PubMed]
- Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 1998;279:563-6. [PubMed]
- Yu H, Spitz MR, Mistry J, et al. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 1999;91:151-6. [PubMed]
- Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 1999;91:620-5. [PubMed]
- MacDonald K, Porter GA, Guernsey DL, et al. A polymorphic variant of the insulin-like growth factor type I receptor gene modifies risk of obesity for esophageal adenocarcinoma. Cancer Epidemiol 2009;33:37-40. [PubMed]
- Ogunwobi OO, Beales IL. Leptin stimulates the proliferation of human oesophageal adenocarcinoma cells via HB-EGF and Tgfalpha mediated transactivation of the epidermal growth factor receptor. Br J Biomed Sci 2008;65:121-7. [PubMed]
- Brochu-Gaudreau K, Rehfeldt C, Blouin R, et al. Adiponectin action from head to toe. Endocrine 2010;37:11-32. [PubMed]
- Lara-Castro C, Luo N, Wallace P, et al. Adiponectinmultimeric complexes and the metabolic syndrome trait cluster. Diabetes 2006;55:249-59. [PubMed]
- Howard JM, Beddy P, Ennis D, et al. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg 2010;97:1020-7. [PubMed]
- Lagergren J, Nyrén O. Do sex hormones play a role in the etiology of esophageal adenocarcinoma? A new hypothesis tested in a population-based cohort of prostate cancer patients. Cancer Epidemiol Biomarkers Prev 1998;7:913-5. [PubMed]
- Armstrong RW, Borman B. Trends in incidence rates of adenocarcinoma of the oesophagus and gastric cardia in New Zealand, 1978-1992. Int J Epidemiol 1996;25:941-7. [PubMed]
- Lepage C, Rachet B, Jooste V, et al. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol 2008;103:2694-9. [PubMed]
- Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2008;100:1184-7. [PubMed]
- Clegg DJ, Brown LM, Woods SC, et al. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006;55:978-87. [PubMed]
- Quinton ND, Smith RF, Clayton PE, et al. Leptin binding activity changes with age: the link between leptin and puberty. J Clin Endocrinol Metab 1999;84:2336-41. [PubMed]
- Quinton ND, Laird SM, Okon MA, et al. Serum leptin levels during the menstrual cycle of healthy fertile women. Br J Biomed Sci 1999;56:16-9. [PubMed]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 2002;42:461-71. [PubMed]
- Morita Y, Iwamoto I, Mizuma N, et al. Precedence of the shift of body-fat distribution over the change in body composition after menopause. J Obstet Gynaecol Res 2006;32:513-6. [PubMed]
- Ainslie DA, Morris MJ, Wittert G, et al. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord 2001;25:1680-8. [PubMed]


