Role of gemcitabine as second-line therapy after progression on FOLFIRINOX in advanced pancreatic cancer: a retrospective analysis
Introduction
Pancreatic adenocarcinoma is a highly lethal cancer, being responsible for about 266,000 deaths per year worldwide. It represents the eighth cancer-related death in men and the ninth in women (1,2). Surgery is the only potentially curative treatment. However, due to the delayed diagnosis of pancreatic cancer, surgery is feasible in only 15% to 20% of patients and, even after resection, the prognosis is very poor.
Systemic chemotherapy offers benefit for advanced pancreatic cancer, improving symptoms and overall survival (OS) when compared to best supportive of care (BSC) (3). Until recently, first-line therapy was gemcitabine-based chemotherapy, with a median OS of 5 to 6 months (3). Based on the phase III PRODIGE 4 trial, which compared FOLFIRINOX (a combination of 5-fluorouracil, oxaliplatin and irinotecan) to gemcitabine as first-line therapy in 342 patients with metastatic disease, FOLFIRINOX showed higher progression-free survival (PFS) and OS. Therefore, FOLFIRINOX became the standard first-line therapy in patients with good performance status (4).
Although recent advances have improved outcomes in the first-line therapy of metastatic pancreatic cancer, all patients will eventually develop disease progression. Treatment options in subsequent lines are limited and there is no standard of care in this setting. Despite the lack of clinical trials reporting the benefit after first-line therapy, gemcitabine-based chemotherapy has been routinely used as second-line therapy in most centers for patients who fail FOLFIRINOX. Based on the growing need to understand the real benefit of gemcitabine as second-line therapy in patients previously treated with FOLFIRINOX, we aim at showing our retrospective experience with gemcitabine as second-line therapy after progression on FOLFIRINOX.
Methods
Patients
Patients diagnosed with advanced pancreatic adenocarcinoma treated with gemcitabine as second-line therapy after progression on first-line FOLFIRINOX at São José Hospital (Beneficência Portuguesa de São Paulo, Brazil) from January 2011 to July 2014 were eligible for analysis. Patients who received at least one cycle of gemcitabine were included. Medical records were retrospectively reviewed after approval by the hospital Research and Ethics Committee.
Eligibility criteria for this retrospective review included histologic diagnosis of metastatic pancreatic adenocarcinoma, progression of disease on FOLFIRINOX as first-line therapy and use of at least once cycle of gemcitabine as second line therapy. Data collection was concluded in September 2014. Gemcitabine-related side effects were not evaluated in this study.
Treatment
Gemcitabine was administered intravenously (IV) at a dose of 1,000 mg/m2 on days 1, 8 and 15 every 4 weeks until disease progression. Imaging evaluation was performed with computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography scan (CT/PET scan), at the discretion of the attending physician. Imaging tests were analyzed by radiologists at our institution according to response evaluation criteria in solid tumors.
Statistical analysis
Demographic and clinical characteristics were summarized by medians and frequencies, as appropriate. The primary end-point was PFS, defined as the time interval between gemcitabine beginning and time of disease progression based on imaging studies or death, whichever occurred first. OS was defined as the time interval between gemcitabine beginning and time of death or last follow-up. PFS and OS were estimated by Kaplan-Meier method. SPSS for Windows version 22.0 was used for data analysis (SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics
Thirty-five patients were initially eligible for this study. Twenty out of those 35 patients received at least one complete cycle of gemcitabine and qualified for the study. Patients’ characteristics are listed in Table 1. Median age was 57 years (range, 43-74 years), and 55% were older than 60 years. Most patients were male (80%), had metastatic disease (60%), and ECOG performance status of 0 or 1 (65%). All patients were treated with FOLFIRINOX as first-line therapy. Only three patients had received prior gemcitabine as adjuvant therapy after surgery.
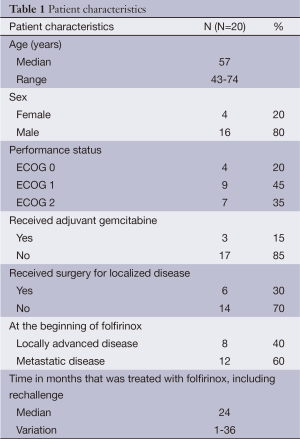
Full table
Treatment
Median time on gemcitabine as second-line therapy was 8 weeks, ranging from 1 to 8 cycles. Posology and dose reductions due to toxicity occurred at each oncologist’s discretion. Most patients (90%) received a dose of 1,000 mg/m2, and 2 patients (10%) received 800 mg/m2 on days 1, 8 and 15 every 4 weeks. All patients (100%) discontinued treatment due to either disease progression or death.
Efficacy
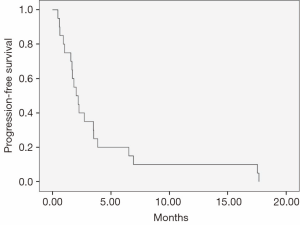
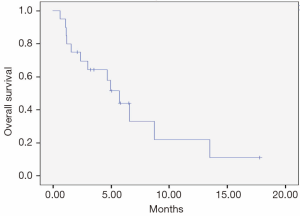
Median PFS and OS were 2.0 months (95% CI, 1.2-2.8) and 5.7 months (95% CI, 3.9-7.4), respectively (Figures 1,2). No treatment-related deaths were reported.
Discussion
Until recently, gemcitabine-based chemotherapy was the standard approach as first-line therapy in advanced pancreatic cancer. This was based on a phase III trial that enrolled 126 patients and randomly assigned them to gemcitabine (1.000 mg/m2 IV weekly for 7 weeks followed by one week off, then weekly for 3 weeks every 4 weeks) or to 5-FU (600 mg/m2 weekly). Gemcitabine showed a clinical benefit of 23.8%, as assessed by improvement in pain (measured by consumption of analgesics and pain intensity), Karnofsky performance status and weight, compared to only 4.8% in the 5-FU/leucovorin group (P=0.0022). Gemcitabine also showed a slight increase in OS (5.65 vs. 4.41 months, P=0.0025) (3).
There are currently two more aggressive regimens which could be considered for first line therapy in patients with advanced pancreatic carcinoma and good performance status based on pivotal phase III trials: FOLFIRINOX (4) and nab-paclitaxel/gemcitabine (5). As nab-paclitaxel is yet not approved in Brazil, FOLFIRINOX is our first line regimen for patients with good performance status. However, patients usually progress through development of resistance due to the presence of sub-populations of resistant cells or stromal changes related to inflammation in the cellular microenvironment (6). Therefore, there is a pressing need for more treatment options beyond first-line therapy.
As far as second line treatment for pancreatic cancer is concerned, OFF (oxaliplatin, 5-FU, leucovorin) is the only regimen evaluated in a randomized trial against placebo in the second-line setting after progression on gemcitabine. The German CONKO trial, which closed prematurely due to low recruitment, randomized 46 patients to receive OFF or BSC, yielding better OS in the chemotherapy group (4.8 vs. 2.3 months; HR 0.45; 95% CI, 0.24-0.83; P=0.008). However, the first-line therapy in the CONKO trial consisted of gemcitabine, which was the only standard regimen at that time (7).
On the other hand, the Canadian multicentre trial PANCREOX did not demonstrate improved outcomes for the addition of oxaliplatin to 5-FU in second-line setting after gemcitabine failure. The study enrolled 108 patients who were previously treated with gemcitabine, and randomized them to receive either mFOLFOX6 (5-FU and oxaliplatin) or infusional 5-FU plus leucovorin. The combination of 5-FU and oxaliplatin did not show a significantly increase in PFS (3.1 vs. 2.9 months, respectively; HR 1.00; 95% CI, 0.66-1.53; P=0.99), and surprisingly resulted in decreased OS (6.1 vs. 9.9 months; HR 1.78; 95% CI, 1.08-2.93; P=0.02) and worse toxicity profile (8).
Recently, a systematic analysis evaluating the role of second-line therapy in advanced pancreatic cancer after progression on gemcitabine was reported. A total of 1,503 patients were included. Among patients treated with second-line chemotherapy (n=1,269), OS was 6 months compared to 2.8 months (P=0.013) in patients who received BSC (n=234). The gemcitabine and platinum-based therapy provided PFS and OS of 4 and 6 months compared with 1.6 and 5.3 months for other regimens, (P=0.059 and 0.10, respectively). The combination of 5-FU and platinum agents obtained a PFS of 2.9 months and an OS of 5.7 months (P=0.60 and 0.22, respectively) (9).
Given the proven benefits of gemcitabine as first-line therapy associated with the absence of a standard second-line regimen for patients who are refractory to FOLFIRINOX, gemcitabine has been consistently used in many services as second-line therapy, despite the lack of clinical trials proving the real benefit of gemcitabine in this setting. We report our experience with gemcitabine in patients who were previously treated with FOLFIRINOX and showed a median PFS of 2 months with a median OS of 5.7 months. Our results compare favorably with the OS data of gemcitabine in the first-line setting, suggesting that patients who progress on FOLFIRINOX may also benefit from second-line treatment.
Whether gemcitabine is the most effective second-line treatment for patients who progress on FOLFIRINOX remains uncertain. In the first-line setting, the combination of gemcitabine and nab-paclitaxel seems superior when compared to single-agent gemcitabine according to the MPACT trial. This study randomly assigned 861 patients with advanced pancreatic cancer to receive gemcitabine alone versus gemcitabine plus nanoparticle albumin-bound paclitaxel as first-line therapy. It showed a significantly increase in OS favoring the combination (5). This data suggests that combined therapy based on gemcitabine plus either a platinum agent or a taxane should be evaluated as second-line therapy in randomized trials.
Our study has important limitations. Its retrospective nature and the small number of patients are the major ones. Moreover, adverse events data could not be evaluated. Despite these limitations, our study is the first, to our knowledge, to report the use of gemcitabine as second-line therapy for patients previously treated with FOLFIRINOX as first line therapy. Besides, the reduced number of patients confirms that only few patients with advanced pancreatic cancer have clinical conditions and performance status to qualify for second-line treatment instead of BSC. Despite these limitations, our study is the first, to our knowledge, to report the use of gemcitabine as second-line therapy for patients previously treated with FOLFIRINOX, the current standard first-line treatment in patients with metastatic pancreatic cancer and good performance status.
Conclusions
Our small retrospective study suggests that gemcitabine is a reasonable treatment option as second-line therapy in patients with advanced pancreatic cancer who progress on FOLFIRINOX. Nonetheless, only a phase III clinical trials comparing gemcitabine versus BSC can evaluate the real benefit of this chemotherapy after progression on FOLFIRINOX.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [PubMed]
- Hamada S, Masamune A, Shimosegawa T. Novel therapeutic strategies targeting tumor-stromal interactions in pancreatic cancer. Front Physiol 2013;4:331. [PubMed]
- Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. [PubMed]
- Gill S, Ko YJ, Cripps MC, et al. PANCREOX: A randomized phase 3 study of 5FU/LV with or without oxaliplatin for second-line advanced pancreatic cancer (APC) in patients (pts) who have received gemcitabine (GEM)-based chemotherapy (CT). J Clin Oncol 2014;32:abstr 4022.
- Rahma OE, Duffy A, Liewehr DJ, et al. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 2013;24:1972-9. [PubMed]


