Expression of SPARC like protein 1 (SPARCL1), extracellular matrix-associated protein is down regulated in gastric adenocarcinoma
Introduction
SPARC-like protein 1 (SPARCL1) is a member of the SPARC family of extracellular matrix-associated proteins, which, in addition to SPARC, includes testicans 1-3, tsc36, QR1, and SMOCs. The SPARC family of proteins is defined by the presence of a highly acidic domain-I, a follistatin-like domain, and an extracellular calcium (EC) binding domain (1). The follistatin-like domain, which shares homology to a repeated domain in follistatin, consists of a Kazal-type serine protease inhibitor region and an EGF domain. The EC binding domain contains a canonical pair of Ca21 binding EF-hand motifs (1). In immunoblots of brain proteins SPARCL1 was detected as a doublet band of 116 and 120 kDa, whereas the molecular mass predicted from amino acid sequence was 70.6 kDa (2). SPARCL1 is down-regulated in many types of cancer cells and may serve as a negative regulator of cell growth and proliferation (3-5). Furthermore, expression of SPARCL1 is associated with the migration of myotomes during stomatogenesis in early mouse embryos and undergoes a rapid down-regulation just before myotome emigration from the somatic environment (6).
In case of gastric cancer (GC) incursion, metastasis, and recurrence are the events leading it to life-threatening aspect which is associated with poor prognosis (7). Even after a remedial resection or adjuvant therapy, nearly 60% of those patients affected succumb to GC (7). In the present study we analyzed the expression of SPARCL1 in gastric adenocarcinoma tissues by immunohisto chemical analysis (ICA) and western blotting (WB).
Materials and methods
Antigen
DNA coding for amino acids 18-451 of human SPARCL1 was cloned in frame with GST and thrombin cleavage site in pGEX4T1 (Amersham Biosciences Corp., NJ, USA). The plasmid was transformed into chemically competent BL-21 (DE3) and induced with 1 mM IPTG for 3 hours; E. coli cells were harvested by centrifugation and subjected to lysis by adding SDS lysis buffer along with lysosome followed by a brief sonication (8). The glutathionine-sepharose bead enrichment was done with cell lysate. The fusion protein was eluted by adding 1ml of elution buffer (50 mM Tris-HCl, 10 mM reduced glutathione, pH 8.0). SPARCL1 was cleaved from GST by thrombin protease (Sigma). The cleaved protein was purified using GSTrap FF 5 mL column (Amersham-Pharmacia Biopharmaceuticals) followed by HiTrap Benzamidine FF 1 mL column (Amersham-Pharmacia Biopharmaceuticals) using AKTA Prime system (GE Health Care). Purified protein was analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and appeared as two bands ~97 and ~120 kDa when stained with Coomassie blue (Sigma). Mass spectrometric analysis of both bands was done were done using peptide (LLAGDHPIDLLLR) from SPARCL 1 as described (9).
Immunization
Five healthy female BALB/c mice (L, R, B, N, 2L) of 6 to 8 weeks old, weighing 25 to 30 gm were obtained from the Animal Breeding Center of IMGENEX India Pvt. Ltd. Animal experiments were performed in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and approved by Institutional Animal Ethics Committee (IAEC). Mice were immunized intra-peritoneally (IP) with 50 µg of recombinant human SPARCL1 emulsified with CFA (Sigma) in a ratio of 1:1 (v/v) (10,11). Another three subsequent boosters (injection) were given on 14th, 28th and 42nd day of immunization with 25 µg of the protein emulsified with IFA (Sigma) at a ratio of 1:1 (v/v). On day 49 the mice were bled and the sera were tested by indirect ELISA. The mouse that showed the highest antibody titer by indirect ELISA was further screened for specificity by western blot. Four days prior to fusion, the selected mouse was given a final booster (intra venous) with 25 µg of protein, final volume of 100 µL with sterile PBS.
Preparation of hybridomas
Splenectomy was performed 4 days after the last immunization and spleen cells were used to prepare hybridomas by standard procedure (12). Spleen cells were fused with F0 myeloma cells at a ratio of 5:1 in polyethylene glycol (PEG). Fused cells were grown in DMEM supplemented with HAT and 10% FBS at 37 °C in 8% CO2. Four weeks later, HAT medium was replaced by DMEM with 10% FBS. Positive hybridomas from ELISA were cloned by the extensive limiting dilution method. After 8 weeks, hybridomas were transferred to fresh DMEM supplemented with 10% FBS.
Screening of hybridomas by ELISA
Hybridoma culture supernatants were screened by ELISA as described elsewhere (13). Briefly, 96-well microtiter plates (Nunc) were coated with 100 ng of recombinant SPARCL1 in carbonate buffer. Culture supernatants were added as primary at proper dilutions and binding was monitored by rabbit anti mouse antibody conjugated to HRP (Jackson’s). TMB (Sigma) was used as chromogenic substrate to detect the color development at 450 nm. Amongst the clones tested, the highest ELISA titer clone (2A4H11) was selected and expanded.
Purification and isotyping of monoclonal anti-SPARCL1 antibody
Monoclonal anti-SPARCL1 (mAb 2A4H11) was purified from the cell culture supernatant by Protein G affinity chromatography. The purity of the preparation was found to be ≥98% as determined by SDS-PAGE and staining by Coomassie blue. The heavy chain and light chain subclass of the anti-SPARCL1 mAb (mAb 2A4H11) was determined by ELISA. For heavy chain determination, hybridoma cell culture supernatant was added to micro-titer wells coated with recombinant SPARCL1 and bound antibody was probed with appropriately diluted rabbit anti-sera to mouse IgG1, IgG2a, IgG2b, IgG3, IgA or IgM (Sigma). The resulting complex was detected by peroxidase conjugated anti-rabbit Ab (Jackson). ABTS [2,2-azino-bis-(3-ethyl-benzthiazoline-6-sulfonic acid), Sigma] was added and the development of color was monitored at 405 nm. The light chain subclass was identified by incubation of mAb 2A4H11 bound to recombinant human SPARCL1 with peroxidase labeled rabbit anti-mouse-k or -l light chain specific immunoglobulin (Sigma). ABTS was added to develop the color and optical density was measured at 405 nm.
Clinical patient specimens collection
Fifteen number of patients (mean age, 52 years; 9 males, 6 females) suffering from GC received surgical resection at Hemlata Cancer Hospital and Research Institute (HCHRI), Bhubaneshwar, India. Gastric adenocarcinoma and adjacent normal tissues were selected post histopathological examination of tissue to identify normal and cancerous tissue was done by an expert pathologist from HCHRI, Bhubaneshwar. All tissue samples were obtained for the present study with patient informed consent, and the use of the human specimens was approved by Institutional Ethics Committee for Human Research (IECHR).
Immunohistochemical labeling
Tissues were removed from fixatives, washed properly in cold 1mM PBS (pH 7.5). Tissues were dehydrated with 30-100% ethanol followed by 2 xylene changes. Tissues were transferred into molten paraffin wax (melting point 58-60 °C, Fisher Scientific, USA) and blocks were prepared. Microtome sections of 5 µm (Leica RM2125, Germany) and fixed into egg albumin-coated glass slides (Blue Star). Formalin fixed paraffin embedded tissue sections and human gastrointestinal tissue microarray slides (Imgenex: IMH-361, Novus, USA) were de-paraffinised and antigen retrieval was done for 20 min in 0.01 mol/L of sodium citrate buffer. Endogenous peroxidase was quenched using hydrogen peroxide. The sections were incubated with primary antibody (6 µg/mL). After rinsing with 1mM PBS (pH 7.5), the slides were incubated with Biotin conjugated goat anti mouse IgG (Jackson’s, USA) at 1:2,000 dilution. The signal was developed using avidin-biotin complex, followed by substrate addition (Vector Nova Red, VECTOR LABS, USA) and counter stained with hematoxylin (SIGMA). Tissue sections were observed using Nikon Eclipse E600, microscope operated using Spot Basic software.
Western blotting (WB)
Tumor and normal gastric adenocarcinoma tissues were homogenized in RIPA buffer (50 mM Tris HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA; 1% Triton-X 100; 1% sodium deoxycholate; 0.1% SDS; 1 mM PMSF) containing protease inhibitor mixture (1× PIC). A total of 20 µg of protein from each tissue was transferred electrophoretically onto PVDF membrane (Merck Millipore, Germany). After transfer of protein, immuno probing was done with anti-SPARCL1, mouse monoclonal antibody at 0.5 µg/mL dilutions as primary antibody followed by horseradish peroxidase-conjugated anti-mouse IgG antibody (Jackson, USA) and ECL immunodetection (Thermo, USA) was used for autoradiography and the chemiluminescent signal was exposed to an X-ray film. Anti-GAPDH (Imgenex: IMG-6665A, Novus, USA) was used as loading control at 1 µg/mL concentration.
Results
Quality control of SPARC like protein 1 (SPARCL1)
In order to generate the antibody, amino acids 18-451 of human SPARCL1 were cloned into pGEX4T1 in frame with GST and thrombin cleavage site. The protein migrated as two bands at approximately 97 and 120 kDa (expected size is 85 kDa) (2). The identity of protein bands were confirmed by mass spectrometric analysis and found to be SPARCL1 (Figure 1).
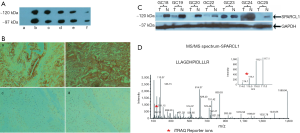
Screening of mice immune sera
All five mice (L, R, B, N, 2L) immunized were ELISA positive. The titer of mice L and N were better than rest of the mice, the binding of mouse N test serum was further tested by WB against recombinant SPARCL1, and observed 120 kDa, 95 kDa band representing SPARCL1 (Figure 1A).
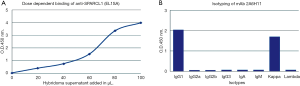
Quality control of mAb 2A4H11 (anti-SPARCL1)
The ability of hybridoma supernatants to bind recombinant human SPARCL1 was analyzed by indirect ELISA. Among all the selected clones, 2A6H11 clone had the highest titer and showed dose-dependent binding to human SPARCL1 by ELISA (Figure 2A). By isotyping it was found that the antibody is IgG1 with kappa chain as shown in Figure 2B. This clone was used for further studies.
Immunohistochemical validation of novel markers of gastric adenocarcinoma: SPARCL1
Down regulation of SPARCL1 was observed in 90% of the tested gastric adenocarcinoma tissues as compared to normal epithelial tissues. As shown in Figure 1B, SPARCL1 was predominantly localized to cytoplasm of the adjacent non cancerous tissues, whereas there was none or negligible staining was observed in the cancerous tissues.
Western blotting (WB) validation of novel markers of gastric adenocarcinoma: SPARCL1
SPARCL1 is observed to be down regulated in all of the eight tested gastric adenocarcinoma tissues as compared to normal epithelial tissues. As shown in Figure 1C, SPARCL1 predominantly gave ~120 kDa bands on adjacent non-cancerous tissues, whereas there was no/lower intensity band was observed in the cancerous tissues.
Discussion
SPARCL1 mRNA is down regulated in many epithelial tumors (3-5,14-16). In addition, SPARCL1, extracellular matrix-associated protein can act as a negative regulator of cell proliferation when over expressed in HeLa 3S cells (5). The down regulation of SPARCL1 in different tumor tissues prompted, to study the expression profile of SPARCL1 in gastric adenocarcinoma, which might play important role in suppression of gastric adenocarcinoma tumor proliferation. To study the expression of SPARCL1 in gastric adenocarcinoma, monoclonal antibody against recombinant human SPARCL1 was developed, with this antibody immunohistochemical analysis and WB analysis was done using gastric adenocarcinoma and adjacent non-cancerous. It was found that SPARCL1 is expressed in normal/adjacent noncancerous tissues whereas it is absent or present in low level in gastric adenocarcinoma tissues irrespective of patient’s age, sex, and stage of cancer, such differential expression of SPARCL1 proteins suggest that loss of SPARCL1 proteins might contribute to the development of gastric adenocarcinoma. And the down regulation of SPARCL1 in GC may be a consequence of gene deletions or/and point mutations leading to frame shifts, premature translation stop, or to amino acid changes that result in a non-functional SPARCL1 protein. Mutations that lead to incorrect splicing of the pre-mRNA could lead to rapid degradation or sequence variations in the promoter might alter the transcription rate of SPARCL1.
In the present study, SPARCL1 protein expression was down regulated in human gastric adenocarcinoma, and was also reported in previous studies, validating our data (17). To our knowledge, this is the first report on the tissue expression of SPARCL1 proteins in human gastric adenocarcinoma tissues from India. Self-sufficiency in growth, loss of growth inhibitory mechanisms, evasion of apoptosis, sustained angiogenesis, immortality and invasion/metastasis are considered hallmarks of cancer (18). However, the consequences of such down regulation in gastric adenocarcinoma development remain unknown.
In breast cancer, downregulation of SPARCL1 was found to be common and frequent event and loss of SPARCL1 expression was significantly associated with lymphatic metastasis and poor grade of breast cancer (19). Similarly, down regulation of SPARCL1 in gastric adenocarcinoma suggests that SPARCL1 might be a novel tumor suppressor gene. Tumor suppressor genes normally regulate cell growth and differentiation in a negative fashion and their functional inactivation in cancerous cells allow survival and cell cycle progression. Besides, SPARCL1 may be a clinically useful biomarker for diagnostic, prognostic and therapeutic values.
It is likely that SPARCL1 expression is regulated in a complex fashion perhaps involving several transcription factors as it has been found for SPARC (20). To identify factors that bind to the promoter sequence of SPARCL1 it will be interesting to perform a yeast one-hybrid screen and to define the binding regions of the transcription factors using DNA footprint assays.
Acknowledgements
The present study was supported by Imgenex India Pvt. Ltd. Bhubaneswar. Authors would like to acknowledge the staff members of Institute of Bioinformatics, Bangalore, India, for their help and support. We would also like to thank all the staff members of Imgenex India Pvt. Ltd. for their continuous help and support throughout the study. Finally, the authors thank Prof. GB Chainy and Dr. A Pandey for their expert guidance and support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yan Q, Sage EH. SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem 1999;47:1495-506. [PubMed]
- Johnston IG, Paladino T, Gurd JW, et al. Molecular cloning of SC1: a putative brain extracellular matrix glycoprotein showing partial similarity to osteonectin/BM40/SPARC. Neuron 1990;4:165-76. [PubMed]
- Nelson PS, Plymate SR, Wang K, et al. Hevin, an antiadhesive extracellular matrix protein, is down-regulated in metastatic prostate adenocarcinoma. Cancer Res 1998;58:232-6. [PubMed]
- Bendik I, Schraml P, Ludwig CU. Characterization of MAST9/Hevin, a SPARC-like protein, that is down-regulated in non-small cell lung cancer. Cancer Res 1998;58:626-9. [PubMed]
- Claeskens A, Ongenae N, Neefs JM, et al. Hevin is down-regulated in many cancers and is a negative regulator of cell growth and proliferation. Br J Cancer 2000;82:1123-30. [PubMed]
- Ringuette M, Rogers I, Varmuza S, et al. Expression of SC1 is associated with the migration of myotomes along the dermomyotome during somitogenesis in early mouse embryos. Dev Genes Evol 1998;208:403-6. [PubMed]
- Milne AN, Carneiro F, O'Morain C, et al. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet 2009;126:615-28. [PubMed]
- Chung CT, Niemela SL, Miller RH. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A 1989;86:2172-5. [PubMed]
- Kalume DE, Peri S, Reddy R, et al. Genome annotation of Anopheles gambiae using mass spectrometry-derived data. BMC Genomics 2005;6:128. [PubMed]
- Steward JP, Ornellas EP, Beernink KD, et al. Errors in the technique of intraperitoneal injection of mice. Appl Microbiol 1968;16:1418-9. [PubMed]
- Miner NA, Koehler J, Greenaway L. Intraperitoneal injection of mice. Appl Microbiol 1969;17:250-1. [PubMed]
- Köhler G, Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol 1976;6:511-9. [PubMed]
- Kulaga H, Sogn JA. A plate-binding elisa for use with hybridoma supernatant fluids. Journal of tissue culture methods 1985;9:151-3.
- Notterman DA, Alon U, Sierk AJ, et al. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res 2001;61:3124-30. [PubMed]
- Esposito I, Kayed H, Keleg S, et al. Tumor-suppressor function of SPARC-like protein 1/Hevin in pancreatic cancer. Neoplasia 2007;9:8-17. [PubMed]
- Kaleağasıoğlu F, Berger MR. SIBLINGs and SPARC families: their emerging roles in pancreatic cancer. World J Gastroenterol 2014;20:14747-59. [PubMed]
- Li P, Qian J, Yu G, et al. Down-regulated SPARCL1 is associated with clinical significance in human gastric cancer. J Surg Oncol 2012;105:31-7. [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [PubMed]
- Cao F, Wang K, Zhu R, et al. Clinicopathological significance of reduced SPARCL1 expression in human breast cancer. Asian Pac J Cancer Prev 2013;14:195-200. [PubMed]
- Vial E, Perez S, Castellazzi M. Transcriptional control of SPARC by v-Jun and other members of the AP1 family of transcription factors. Oncogene 2000;19:5020-9. [PubMed]


