Thirty days post-operative mortality after surgery for colorectal cancer: a descriptive study
Introduction
Colorectal cancer is one of the most occurring malignancies, at least in the Western world (1-4). The only curative option is surgery and post-operative mortality should be minimized. Post-operative events ultimately leading to death of the patient, of course are very frustrating for the entire surgical and medical team. Since many patients with colorectal cancer are older (5), it is to be expected that an increasing number of patients have co-morbidity rendering any operation more hazardous. Even in cases of successful surgery, patients can die due to complications induced by their co-morbidity.
Many pre-operative scoring systems are used in order to identify patients at risk. Although data on post-operative mortality of colorectal surgery are well-known, little is known about the effect of co-morbidity and the direct causes of post-operative mortality. A large study from the Department of Surgery from the Zaans Medical Centre clearly showed that the strongest predictors of in-hospital mortality were emergency surgery, tumor stage, age, pulmonary failure and cardiac failure (6).
But data on post-operative death due technical failure of surgery are not known. For this reason a study was done in consecutive patients operated upon because of colorectal cancer in order to identify causes of mortality in the post-operative period, and more specifically assess the technical failure of the operation.
Patients and methods
All consecutive patients who underwent surgery for colorectal cancer in the Zaans Medical Centre, the community hospital of the Zaanstreek region in the Netherlands, in the time period of 2002-2008, were included. An extensive chart review was done in order to identify co-morbidity of each individual patient. Co-morbidity for each patient was expressed as the Charlson co-morbidity score. This score adds up points for each accompanying illness or condition together with a score for age. The diagnosis of cancer obviously was excluded in the calculation of this score (7-9).
The post-operative course of each surgical patient was studied and the cause of death within 30 days after surgery was determined. Age of the patients, as well as localization of the tumor and cancer stage was noted as well.
Patients were divided into two groups. Patients in group 1 died within 30 days after surgery (the post-operative mortality group), and patients in group 2 survived (the majority being operated upon) for longer than 30 days.
Statistical analysis was done with Chi-square test for contingency tables and t-test. A value below 0.05 was considered statistically significant.
Results
A total of 392 patients with colon cancer and 145 patients with rectal cancer were diagnosed. Fifty nine patients with colon cancer and 34 with rectal cancer did not undergo surgery mostly because of metastatic disease (stage 4). Twenty three out of 333 patients (6.9%) with colon cancer died within the post-operative period of 30 days. This was 6 out of 112 patients (5.3%) with rectal cancer.
Patients in group 1 with colon cancer were operated with curative intent in 12 cases and for palliative reasons in 11. This was 4 and 2 for rectal cancer respectively.
Patients in group 2 underwent surgery with curative intent in 295 cases of colon cancer, and for palliation in 74. For rectal cancer this was 105 and 1 respectively.
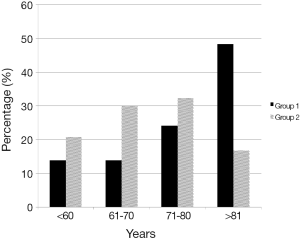
Patients in group 1 were significantly older than patients in group 2 (P<0.001), especially octogenarians were overrepresented (Figure 1). However, in sub-analysis this was especially true for patients with colon cancer. The numbers in cases of rectal cancer did not reach statistical significance. But the number of patients who died was too low to reach definite conclusions.
Patients in group 1 with colon cancer also significantly had more often a higher stage compared with patients in group 2 (P=0.03) (Figure 2, Table 1). There was no difference in tumor localization.
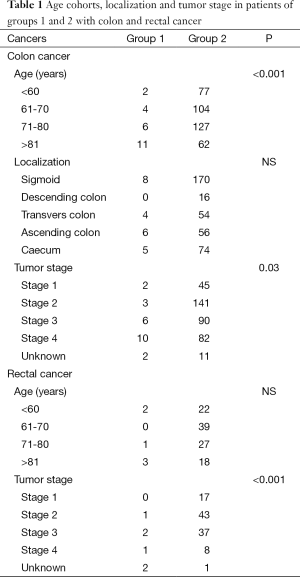
Full table
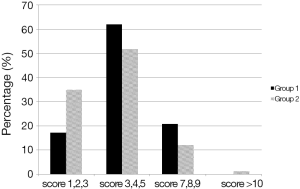
The mean Charlson co-morbidity score for patients with colon cancer in group 1 was 5.17 (SD 1.57, range, 1-8), and for rectal cancer 4.83 (SD 2.32, range, 2-7). Although there was a tendency towards a higher Charlson co-morbidity scores in group 1 this was not significant (Figure 2).
In patients with colon cancer of group 1, 12 died as a direct consequence of surgical complications [acute surgery because of acute abdomen with peritonitis (n=3), post-operative bleeding requiring reoperation (n=3), anastomotic leakage requiring relaparotomy (n=5), abscess formation resulting in irreversible septic shock (n=1)]. Eleven patients died due to complications induced by their pre-existing co-morbidity [cardiovascular (n=3), pulmonary complications (n=2), complications of pre-operative palliative chemotherapy (n=2), septicemia not related to the operation with multi organ failure (n=3), acute rupturing aneurysm (n=1)]. Causes of post-operative death in patients with rectal cancer were: anastomotic leakage (n=1), acute coronary problems (n=2), acute surgery because of perforation with peritonitis (n=2), and septicemia post radiation therapy.
Discussion
The present study shows that 44% of patients who died in the post-operative period, died because of the direct technical complications of surgery. This is 2.2% of the total population of patients who underwent surgery. The remainder died because of the effects of existing co-morbidity. The five patients presenting with an acute perforation and peritonitis can be judged as indirect complication of surgery. In other words, without surgery these patients would have died anyway. Hence, the number dying of direct technical failure (anastomotic leakage, bleeding) is rather low. Of course, this is a somewhat biased conclusion. Without surgery these complications would not have occurred. On the other hand, patients in good clinical condition possibly have the strength to survive the complications due to technical failure. Due the application of post-operative prophylactic anticoagulant therapy no patient died because of thromboembolism.
In a large population based study in the Netherlands (25,591 patients undergoing colorectal resections in 92 hospitals), postoperative mortality rates ranged between 0% and 8.8%. Case-mix appeared an important cause of differences between hospitals. In addition, a large proportion of variation in mortality was due to chance (10).
Functional health status predicts postoperative outcomes. A detailed preoperative evaluation, providing an optimization period before surgery if necessary, is mandatory (11). Of course, the entire surgical team tries to minimize the risks of surgery in the individual patient. The introduction of laparoscopic techniques was a step forward in reducing mortality, particularly for the elderly (12). If mortality occurs evaluation is needed in order to determine whether shortcomings in technique or care have occurred. Due to these post-operative evaluations surgical mortality decreased in the past decades (13,14). But, patients still die without the surgical team being directly responsible.
The Charlson co-morbidity score is a well-known score for co-morbidity. Morris et al. showed that post-operative mortality increased in patients aged >80 years with a Charlson co-morbidity score ≥3, or with distant metastases, and in case of operative urgency (13). It is to be expected that patients undergoing acute surgery because of an acute abdomen do die because of the critical clinical situation already present when surgery starts. The urgency character of the surgery does not allow proper rebalancing of the patients with respect to co-morbidity. This was also the case in the five patients in the present study presenting with an acute abdomen with peritonitis.
Surgical complications can be prevented by a proper technique and the severity of complications can be minimized by close post-operative surveillance in order to detect anastomotic leakage or bleeding early. Overall mortality rate is higher in patients after relaparotomy because of anastomotic leakage (15). There appear to be several risk factors for this complication such as extensive tumor resection, emergency surgery, transverse colon resection, or subtotal colectomy (16).
It is to be expected that patients who are healthy a have better outcome of anastomotic leakage compared with patients with cardiovascular or pulmonary problems. However, on the other hand a recent study showed that patients operated in hospitals with a higher reoperation rate did not have higher mortality rates (17).
High age, especially above 80, is an important factor in post-operative mortality in cases of colorectal cancer. This is in accordance with the literature (18). Factors that negatively influence results of surgery are diabetes and pre-existing cardiac pathology. The co-morbidity is an important factor in mortality because the majority of patients in this study died due to events related to their co-morbidity. This can be a case of bad luck, since there was no difference in co-morbidity with patients who survived the post-operative period.
The clinician has to make difficult decisions, especially in the older patient with colorectal cancer. One has to be aware of under treatment in healthy and fit older patients, while on the other hand overtreatment in the vulnerable or frail patient can lead to unacceptable postoperative outcomes with high mortality or persistent disability (19). One has to keep in mind that the 1-year mortality after colorectal cancer surgery is high especially in older patients with a short life expectancy. A recent study in the Netherlands showed that 13% of patients died within the first postoperative year. Death was attributed to colorectal cancer in only 75% of patients (20).
It is concluded that pre-existing co-morbidity is an important factor in post-operative mortality. The number of patients dying as a direct result of technical failure of the operation is rather low.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Rim SH, Seeff L, Ahmed F, et al. Colorectal cancer incidence in the United States, 1999-2004: an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1967-76. [PubMed]
- Dutch Cancer registration. Available online: www.cijfersoverkanker.nl
- Integral Cancer Institute Amsterdam. Available online: www.ika.nl
- Loffeld RJ, Dekkers PE, Flens M. The incidence of colorectal cancer is decreasing in the older age cohorts in the zaanstreek region in the Netherlands: an age-cohort effect. ISRN Gastroenterol 2013;2013:871308.
- van der Sluis FJ, Espin E, Vallribera F, et al. Predicting postoperative mortality after colorectal surgery: a novel clinical model. Colorectal Dis 2014;16:631-9. [PubMed]
- Kastner C, Armitage J, Kimble A, et al. The Charlson comorbidity score: a superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis 2006;9:270-4. [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [PubMed]
- Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg 2004;8:1061-7. [PubMed]
- Henneman D, van Bommel AC, Snijders A, et al. Ranking and rankability of hospital postoperative mortality rates in colorectal cancer surgery. Ann Surg 2014;259:844-9. [PubMed]
- Isik O, Okkabaz N, Hammel J, et al. Preoperative functional health status may predict outcomes after elective colorectal surgery for malignancy. Surg Endosc 2015;29:1051-6. [PubMed]
- Hamaker ME, Schiphorst AH, Verweij NM, et al. Improved survival for older patients undergoing surgery for colorectal cancer between 2008 and 2011. Int J Colorectal Dis 2014;29:1231-6. [PubMed]
- Morris EJ, Taylor EF, Thomas JD, et al. Thirty-day postoperative mortality after colorectal cancer surgery in England. Gut 2011;60:806-13. [PubMed]
- Rutegård M, Haapamäki M, Matthiessen P, et al. Early postoperative mortality after surgery for rectal cancer in Sweden, 2000-2011. Colorectal Dis 2014;16:426-32. [PubMed]
- Mik M, Magdzinska J, Dziki L, et al. Relaparotomy in colorectal cancer surgery--do any factors influence the risk of mortality? A case controlled study. Int J Surg 2014;12:1192-7. [PubMed]
- Bakker IS, Grossmann I, Henneman D, et al. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014;101:424-32; discussion 432. [PubMed]
- Henneman D, Dekker JW, Wouters MW, et al. Benchmarking clinical outcomes in elective colorectal cancer surgery: The interplay between institutional reoperation- and mortality rates. Eur J Surg Oncol 2014;40:1429-35. [PubMed]
- de Vries S, Jeffe DB, Davidson NO, et al. Postoperative 30-day mortality in patients undergoing surgery for colorectal cancer: development of a prognostic model using administrative claims data. Cancer Causes Control 2014;25:1503-12. [PubMed]
- Ugolini G, Ghignone F, Zattoni D, et al. Personalized surgical management of colorectal cancer in elderly population. World J Gastroenterol 2014;20:3762-77. [PubMed]
- Dekker JW, Gooiker GA, Bastiaannet E, et al. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol 2014;40:1481-7. [PubMed]