|
Review Article
FDG PET imaging in the staging and management of gastric cancer
Shane Hopkins1, Gary Y Yang2
1Department of Radiation Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA;
2Department of Radiation Medicine, Loma Linda University Medical Center, Loma Linda, California, USA
Correspondence to: Shane Hopkins, MD. Roswell Park Cancer Institute, Department of Radiation Medicine, Elm & Carlton Streets, Buffalo, New York 14263, USA. Tel: (716) 845-3173; Fax: (716) 845-7616. E-mail: shane.hopkins@roswellpark.org
|
|
Abstract
Gastric cancer is a leading cause of cancer death worldwide. Complete resection offers the only chance for permanent control, and accurate staging and evaluation of treatment response are crucial for appropriate management. Positron Emission Tomography (PET) is increasingly used to complement anatomic imaging in cancer management. PET use in gastric cancer has been limited by 1) some gastric histologies are not PET avid, 2) spatial resolution limits the ability to distinguish between primary tumor and compartment I or II lymph nodes, and 3) the lack of a unified criteria in how to interpret PET for management decisions. New criteria have been proposed establishing response metrics in the utilization of PET. More study is needed to support these criteria in routine practice and establish the place of PET in the staging and management of gastric cancer.
Key words positron emission tomography; gastric cancer; tumor staging
J Gastrointest Oncol 2011; 2: 39-44. DOI: 10.3978/j.issn.2078-6891.2010.004
|
|
Introduction
Gastric cancer is one of the most prevalent cancers worldwide and is a leading cause of cancer mortality. In several Eastern countries, gastric cancer is the most common and deadly ma l ignanc y. In the Western Hemisphere gastric cancer incidence has been decreasing while esophageal and gastroesophageal junction cancers have increased (1,2). In the West, gastric cancers are typically distributed in the proximal lesser curvature, in the cardia, and in the GE junction; this distribution has been changing from a more distal distribution in the past and differs from Eastern countries with higher incidence. More than 80% of gastric cancer patients in the West are diagnosed at an advanced stage resulting in poor prognosis (3). Complete resection of gastric cancer is the only method of achieving permanent control. However, surgeries can be morbid and futile in patients who have advanced disease, making appropriate staging and characterization of disease burden of paramount importance. Staging of gastric cancer typically makes use of a variety of imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasounds (EUS), and combined positron tomography (PET-CT), as well as laparoscopic staging and cytogenetic analysis of peritoneal fluid in appropriate patients (4-6). The value of PET-CT has been of increasing interest among clinicians and data has supported its increased use in the detection, staging, and management of a variety of malignancies. During and after therapy, PET-CT may be useful in determining response to chemotherapy. It may be helpful for restaging and diagnosing recurrence at an earlier time or with greater certainty. This paper will address the potential uses of PET-CT specifically within the management of gastric cancer.
|
|
Background
PET is performed by injecting a patient with a radiolabeled tracer which is concentrated by the body in certain metabolically active tissues. As radioactive decay occurs, emissions are measured with a scanner and a threedimensional image representing relative uptake of the tracer is produced. 2-[fluorine 18] fluoro-2-deoxy-D-glucose (FDG) labeled glucose is used most frequently as the tracer, and this paper will assume the use of FDG unless otherwise indicated. As f luorine-labeled glucose is transported into metabolically active cells, it is phosphorylated and trapped, ensuring that continued dissipation and transport do not dilute the signal. These biochemical properties make FDGPET a useful modality for measuring glucose demand as a surrogate for metabolically active tissues such as cancer. In several gastric cancer histologies, however, the metabolic differential between tumor and normal tissue is not as stark as with other malignancies, making the conceptual utility of PET less clear. Mucinous carcinoma, signet ring cell carcinoma, and poorly differentiated adenocarcinomas typically have less prominent FDG uptake (7, 8). Obtaining a PET scan nearly simultaneously with a CT scan using a dual gantry machine allows for registered images represent ing both anatomic and metabol ic properties. The registration is not perfect because the time of image acquisition is longer for PET than the CT portion of the imaging, but obtaining both image sets without moving the patient does provide a more accurate registration while minimizing deformation on overlay. Registration issues may be more pronounced in the GI tract considering the frequent internal daily motion of the organs.
|
|
Staging
The American Joint Committee on Cancer (AJCC) staging system is widely used for the characterization of disease burden and prognosis in gastric cancer. Based on a TNM system, the 7 th edition of AJCC guidelines designate tumor characteristic staging (T) as follows: T1 when tumor invades lamina propria or muscularis mucosae, T2 when tumor invades muscularis propria, T3 when tumor penetrates subserosal tissue without further invasion, and T4 when tumor invades visceral peritoneum or adjacent structures (9). Because surgical treatment is a major prognostic factor, effort to accurately determine the invasiveness of a gastric lesion is crucial. CT-determined T staging agreed closely with pathologic staging in early studies but was subsequently shown to have disappointing accuracy. EUS is a more accurate method for determination of pre-operative T stage and was directly compared with CT in a study by Botet (10). However, evolving technologies produce ever-increasing resolution of CT imaging, and thinsection scans with multiplanar reformation and contrast suggest the comparative value between CT and EUS is not static (11). Regardless of the imaging modality used, loss of the fat plane between a gastric mass and adjacent organs is suggestive of invasion. For this reason, PET imaging is not particularly helpful in determining the T stage. The resolution of PET is limited by volume averaging of metabolic signal, with prominent uptake averaged across several millimeters—a distance too great to give confidence when assessing barrier invasion on the surface of organs.
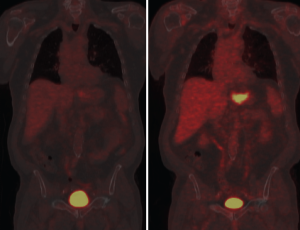
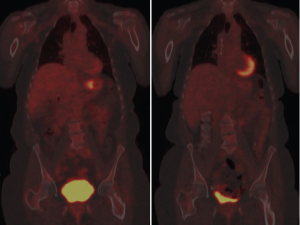
N stage in the 7 th edition of AJCC staging criteria is based on number of positive nodes with some changes from the previous editions. N1, N2, and N3 represent positivity in 1-2, 3-6, and 7 or more nodes respectively. Earlier staging criteria included nodal location as an objective criterion for staging. The Japanese Research Society for Gastric Cancer divides gastric nodes into four compartments, each compartment progressively more removed from the stomach (12). A D1 lymphadenectomy includes resection of compartment 1 lymph nodes (perigastric nodes at stations 1-6) while a D2 resection also removes compartment 2 (stations 7-11) and is the standard surgical procedure in high prevalence countries. D3 and D4 lymphadenectomies include their respective compartments. AJCC criteria designates involvement of hepatoduodenal, retropancreatic, mesenteric, and para-aortic nodes (i.e., compartment III and IV) as distant metastases (9). CT criteria for lymph node metastases include size, shape, central necrosis and heterogeneity (13, 14). When these characteristics are present there is a strong correlation with metastatic involvement. However, CT sensitivity suffers because a small tumor burden in a lymph node is unlikely to produce the morphological changes sufficient to satisfy CT criteria. In concept, PET seems an excellent adjunct therapy to detect these anatomically small but potentially metabolically active focuses of metastatic disease. However, the relatively poor spatial resolution of PET makes it less effective because of the difficulty of distinguishing compartment I and II nodes from the primary tumor itself. The real value of PET may be in the detection of "distant" metastatic disease in compartments III and IV and not amenable to surgical resection with a standard D2 lymphadenectomy. Identification of further spread with PET imaging may influence surgical planning for a more aggressive lymphadenectomy or the decision to avoid surgery altogether as futile and unnecessarily morbid (15). Solid organ metastasis from the stomach occurs most commonly in the liver via hematogenous dissemination through the portal vein (16, 17). Lymphatic and peritoneal dissemination are also common pathways of spread in gastric malignancy. Although distant metastases are frequently detectable using contrast CT, PET is perhaps most useful in the detection of these distant sites of solid organ metastases. A meta-analysis by Kinkel designated PET as the most sensitive noninvasive imaging modality for this purpose (18). Because radio-tracer is distributed throughout the body, larger volumes can be more easily scanned than is practical with CT. Peritoneal dissemination is a poor prognostic factor. Detection of peritoneal metastases may change the surgical strategy from curative to palliative or deter the surgeon from laparotomy altogether. Increasingly sophisticated CT scans facilitate diagnosis of peritoneal metastases prior to visual inspection during surgery. PET may give additional sensitivity to CT. Diffuse uptake of tracer that obscures the serpiginous outline of the bowel may be an indicator of peritoneal metastases, as well as discrete areas of local uptake along areas within the peritoneal cavity that are otherwise anatomically unexplained (i.e. outside expected nodal stations or solid viscera) (11).
|
|
Response to therapy
PET may predict response to preoperative chemotherapy in gastric cancer. Ott et al. showed that a 35% decrease in uptake between pre-chemotherapy and PET scan taken 2 weeks after initiation of therapy predicted response with accuracy of 85%. Two year survival rate was 90% in responders and 25% in non-responders using this criteria with p=0.002 (19). Uptake decrease during therapy is a continuous variable and different thresholds have been determined by other investigators. For example, Shah et al found that a 45% cutoff comparing uptake after 35 days was the best value to separate responders from nonresponders and predict outcome (20). In evaluating response to treatment for esophageal carcinoma, studies have shown marked variability (from 10-80%) in the cutoff values determined retrospectively, and it seems likely that gastric cancer may have comparable variability (21). Wahl et al. have proposed a PET Response Criteria in Solid Tumors (PERCIST) analogous to and intended to eventually supercede other anatomic tumor response metrics such as the World Health Organization (WHO) criteria and multiple versions of the Response Evaluation Criteria in Solid Tumors (RECIST) (22). Wahl notes that both qualitative and quantitative approaches have been made in using PET results for response assessment. Because statistically significant variability between SUV values is typical even when tested and retested under careful control, PERCIST criteria proposes a 30% or greater decline as indicative of "medically relevant beneficial changes". Per the criteria, normal reference tissue values are designated within a scan by using a consistent protocol based on regions of interest in the liver and the most active tissues. Wahl suggests that the PERCIST criteria be used as a starting point for clinical trials and clinical reporting. This seems wise as the ad hoc approach to defining PET response has resulted in a body of work that is fragmented to the point of poor relevance. Many gastric cancers are not PET avid and repeat imaging will not provide additional useful imaging in these patients. Wahl recommends the use of RECIST 1.1 in such cases. Ott et al grouped patients with non-avid tumors as similar in prognosis to metabolic non-responders, that is, biologically unfavorable with poorer prognosis. Metabolic responders had a 69% histopathologic response rate while metabolic non-responders had only a 17% histopathologic response rate, similar to the 24% histopathologic response rate of the non-avid group. Survival was also similar between the non-avid group and the non-responding group while significantly different from the responding group (19). In addit ion to sug gest ing response c r iter ia and prognosis groupings, Kim et al. have compared FDG-PET to f luorothymidine (FLT)-PET with interesting results. FLT-PET had a higher sensitivity than FDG-PET and Ott suggests that it may provide a useful adjunct by providing a quantitative assessment of proliferation. While limited work using other radionuclides has been done, the potential for better clinical relevancy makes this area of investigation particularly interesting (23).
|
|
Recurrent disease
Disease recurrence frequently occurs locally in sites that have lost characteristic anatomic features due to surgery. In such cases early detection may allow for better salvage therapy and may be assisted with the use of PET. Glucose metabolism is typically low in scar tissue and high in recurrent tumor. CT remains central in the characterization of post surgical changes and post-treatment monitoring, however, equivocal findings can be better characterized with the added metabolic information of PET. Unfortunately, the same limitations of PET previously discussed apply in this circumstance; specifically, only certain histologies exhibit sufficient uptake necessary for useful sensitivity, and spatial resolution is limited by the current technological limitations of the modality.
De Potter et al. found a longer survival in a cohort of patients with recurrent disease who were PET-negative than their recurrent counterparts with PET-positive disease. However, de Potter warns that the poor sensitivity and low negative predictive value makes PET inappropriate for screening during follow up; rather, PET can provide important information regarding prognosis in patients with recurrence (24). Sim et al. found that the sensitivity and specificity of PET was similar to CT in all sites of recurrence except peritoneum, where it was less sensitive (25). |
|
Conclusion
PET is a promising modalit y with increasing use across a wide variety of malignancies. It is increasingly used in GI cancers as an adjunct in both staging and management decisions. Per NCCN and other consensus guidelines, PET may be used as an option for greater specificity in characterizing suspected disease in gastric cancer; however, anatomic imaging remains the standard recommendation. Some data supports the use of PET in gastric cancer staging, particularly in characterizing distant metastases or lymphatic metastases beyond compartment I or II. Additional work is needed to refine the proposed PERCIST criteria and to find the best parameters of continuous variable for the use of PET in gastric and other GI malignancies.
|
|
References
- Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991;265:1287-9.[LinkOut]
- Yang GY, Ott K. Accomplishments in 2008 in the management of esophageal cancer. Gastrointest Cancer Res 2009;3:S53-7.[LinkOut]
- Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev 2000;26:243-55.[LinkOut]
- Abdalla EK, Pisters PW. Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol 2004;31:513-29.[LinkOut]
- Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: A systematic review. J Clin Oncol 2007;25:2107-16.[LinkOut]
- Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol 2004;31:530-41.[LinkOut]
- Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: Correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003;30:288-95.[LinkOut]
- Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med 2003;44:690-9.[LinkOut]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging handbook: From the AJCC cancer staging manual. 7th ed. Springer; 2010.
- Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, et al. Preoperative staging of gastric cancer: Comparison of endoscopic US and dynamic CT. Radiology 1991;181:426-32.[LinkOut]
- Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, et al. CT and PET in stomach cancer: Preoperative staging and monitoring of response to therapy. Radiographics 2006;26:143-56.[LinkOut]
- Nishi M, Omori Y, Miwa K, editors. Japanese research society for gastric cancer (JRSGC): Japanese classification of gastric carcinoma. 1st English ed. Tokyo, Japan: Kanehara; 1995.
- D'Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: Correlation with pathological findings. A prospective study of 107 cases. Eur Radiol 2000;10:1877-85.[LinkOut]
- Fukuya T, Honda H, Hayashi T, Kaneko K, Tateshi Y, Ro T, et al. Lymph-node metastases: Efficacy for detection with helical CT in patients with gastric cancer. Radiology 1995;197:705-11.[LinkOut]
- Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 2005;103:2383-90.[LinkOut]
- Gore RM. Gastric cancer. Clinical and pathologic features. Radiol Clin North Am 1997;35:295-310.[LinkOut]
- Miller FH, Kochman ML, Talamonti MS, Ghahremani GG, Gore RM. Gastric cancer. radiologic staging. Radiol Clin North Am 1997;35:331-49.[LinkOut]
- Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): A meta-analysis. Radiology 2002;224:748-56.[LinkOut]
- Ott K, Herrmann K, Krause BJ, Lordick F. The value of PET imaging in patients with localized gastroesophageal cancer. Gastrointest Cancer Res 2008;2:287-94.[LinkOut]
- Shah MA, Yeung H, Coit D, Trocola R, Ilson D, Randazzo J, et al. A phase II study of preoperative chemotherapy with irinotecan(CPT) and cisplatin(CIS) for gastric cancer(NCI 5917): FDG-PET/CT predicts patient outcome. J Clin Oncol 2007;25:4502.
- Hopkins S, Yang GY. Positron emission tomography’s utility in esophageal cancer management. J Thorac Dis 2009;1:29-33.
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S-50S.[LinkOut]
- Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging 2006;33:148-55.[LinkOut]
- De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging 2002;29:525-9.[LinkOut]
- Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer 2009;9:73.[LinkOut]
Cite this article as:
Hopkins S, Yang G. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol. 2011;2(1):39-44. DOI:10.3978/j.issn.2078-6891.2010.004
|