Multimodality therapy of rectal gastrointestinal stromal tumors in the era of imatinib—an Indian series
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. They are considered as neoplastic derivatives of interstitial cells of Cajal which are located in myenteric plexus of gut wall (1). Though any part of the gastrointestinal tract may be affected, stomach remains the most common sub site. Most characteristic feature of GIST is the presence of activating mutations in genes for the trans-membrane receptors c-KIT or PDGFRA (>90% cases) (2). Surgery with histologically negative margins remains the mainstay of treatment of non metastatic GIST. Lymph node dissection is not mandatory as spread via lymphatics is considered very rare (3). Imatinib is a selective tyrosine kinase inhibitor targeting the c-KIT or PDGFRA activated GISTs (4). Adjuvant therapy after complete surgical resection has been shown to significantly improve survival in cases of GISTs with a high risk of recurrence (5). Optimum duration of imatinib in the adjuvant setting has been established as 3 years by the Scandinavian German adjuvant trial (6). Tumor size and mitotic index of activity described as the number of mitoses per 50 HPF are considered as the two most important prognostic factors for GISTs.
Rectal GIST accounts for 5% of all GISTs affecting the gastrointestinal system and 0.1% of all the rectal tumors (7).The overall prognosis of rectal GIST is found to be worse compared to GIST arising from other sub sites like stomach (8). Rectal GIST offers special surgical challenge owing to larger size compared to rectal adenocarcinoma as well as its proximity to the sphincter and dense adherence to the pelvic floor. As a result, neoadjuvant therapy with imatinib assumes greater role in rectal GISTs compared to any other sub sites. There are no evidence based recommendations on the treatment of rectal GIST because of its rarity. We report early results of rectal GIST treated at a tertiary care cancer center in India.
Materials and methods
This is a retrospective review of 13 cases of GIST of the rectum diagnosed between January 1, 2010 and June 30, 2015 at Tata Memorial Centre, Mumbai, India. Following a detailed history and physical examination, all patients underwent a complete colonoscopy with biopsy. Loco regional staging was achieved with a baseline MRI pelvis. Contrast Enhanced Computed Tomography (CECT) of the thorax and abdomen was performed to rule out metastatic disease. All treatment decisions were taken by a multidisciplinary team (MDT) comprising of a colorectal surgeon, a gastroenterologist, a pathologist medical oncologist and a radiologist.
Neoadjuvant imatinib was administered to all patients with a threatened CRM and/or when sphincter saving surgery was not possible on initial imaging. Patients receiving neoadjuvant therapy were evaluated for response with MRI pelvis at 3 months interval. Those with favorable response were offered surgery whereas those with either stable disease or need for permanent stoma were continued on neoadjuvant therapy for at least one year. Extent of surgery performed depended on the preoperative MRI pelvis with sphincter preservation attempted whenever it was considered oncologically safe. Post operatively all the patients were treated with adjuvant imatinib for 3 years. Patients were followed up after surgery at 3 monthly intervals for first 3 years, at 6 monthly intervals for next 2 years and annually thereafter. At each follow up, history and clinical examination was performed and the need for imaging was based on the presenting symptoms. Patients who refused surgery after neoadjuvant therapy were continued on imatinib therapy till they developed progressive disease or untoward Imatinib related side effects.
Parameters assessed regarding perioperative imainib therapy included duration of neoadjuvant therapy, response to neoadjuvant therapy and treatment related toxicity. Perioperative outcomes included intraoperative blood loss, length of hospital stay, 30 day post-operative morbidity and mortality. Post operative morbidity was graded according to Clavien-Dindo system and only grade 3/4 complications were included in the analysis. The oncological adequacy of the procedure was assessed with evaluation of the circumferential resection margin (CRM) positivity and distal resection margin involvement. Disease free survival (DFS) was calculated from the time of start of treatment to the time of last follow-up or clinical evidence of recurrent or metastatic disease. Overall survival (OS) was defined as the time of start of treatment to the last follow-up or patient death. Survival curves were plotted using the Kaplan–Meier method. Statistical analysis was performed using SPSS statistical software, version 18.0.
Results
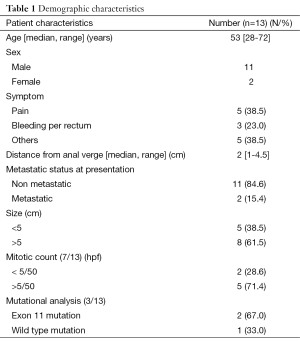
Baseline characteristics of all 13 patients are shown in Table 1. Unlike rectal adenocarcinoma, perianal pain was the predominant presenting symptom. All the 13 patients were CD 117 and DOG 1 positive. Details of the mitotic count were available in 7 patients only. Mutational analysis of c kit was available for only three patients since it was not performed at our institute before the year 2013. Two of these patients had mutation in exon 11 whereas the other had wild type mutation. Among the 2 metastatic rectal GIST patients, one had a solitary metastatic deposit in the right lobe of liver which disappeared after the neoadjuvant therapy whereas other patient had a solitary metastatic deposit in the iliac crest. In view of low systemic load of the disease, both were treated with curative intent.
Full table
Imatinib therapy
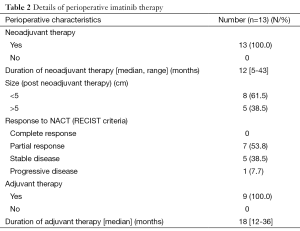
All the patients included in the study received neoadjuvant imatinib therapy (Table 2). Indication for neoadjuvant therapy was large size (>5 cm) in 8 patients and the proximity to pelvic floor in the other 5 patients. None of the patients experienced any grade 3/4 toxicity due to imatinib. Seven patients had partial response whereas 5 patients had stable disease and one patient had progressive disease. The latter patient developed lung and liver metastasis within 6 months of imatinib therapy and patient was not willing for any further investigations or therapy and hence was declared as best supportive care.
Full table
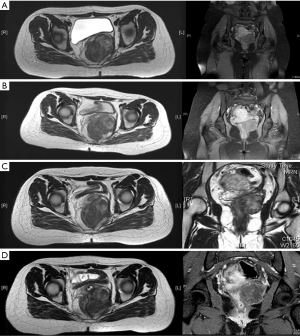
Among the 5 patients with stable disease, four patients were very keen on sphincter preservation. Among these, one patient underwent intersphincteric resection and had complete resection. For two other patients, dose of imatinib was increased to 800 mg OD after stable disease for 12 months with 400 mg OD of imatinib. However they had stable disease even after 6 months of dose escalation and hence were offered abdomino perineal resection (APER) (Figure 1). A fourth patient refused surgery in view of the need for permanent stoma and hence has been continued on imatinib. After 3 years of therapy, he has stable disease and is still not willing for surgery. Last patient is on neoadjuvant imatinib for extended duration.
Among the seven patients with partial response, six patients were successfully treated with surgery whereas one patient refused surgery in view of need for the permanent stoma. The latter patient had tumor at 2 cm from anal verge with involvement of levators and hence sphincter preservation was not feasible. He has been on imatinib for 4 years now, is symptom free and without any drug related toxicity.
Surgical management
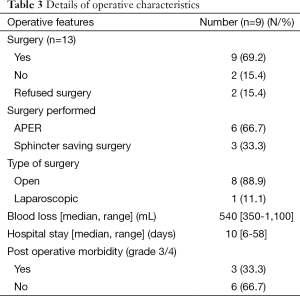
Among 13 patients, nine underwent surgery after neoadjuvant therapy (Table 3). Among these nine patients, three patients underwent intersphincteric resection (33.3%) whereas APER was performed in the rest of the patients. None of the lesions were amenable for local resection even after neoadjuvant therapy. Two patients developed perineal wound complications and both required debridement with secondary suturing under general anesthesia. A third patient had developed hemoperitoneum and was re explored twice for the same.
Full table
Oncological outcomes
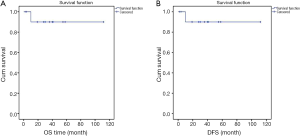
Histopathological features are shown in Table 4. Extent of resection was R0 in 7 patients and R1 in 2 patients (in view of positive CRM). The median follow-up period was 34 months from the time of start of neoadjuvant therapy (range, 8-62 months). One patient developed distant metastasis while on imatinib therapy as mentioned previously. Otherwise none of the patients developed either local recurrence or distant metastasis. Median OS or DFS was not reached in the entire cohort (Figure 2A,B).

Full table
Discussion
GIST of the rectum is uncommon, comprising only 5% of all GISTs. Surgical excision with negative margins remains the mainstay of treatment (9). Local excision is the preferred treatment for early lesions situated in mid or low rectum (10). Approach for local excision may be trans-anal or trans-sacral or trans-vaginal (11-13). In selected patients, these approaches enable safe removal of the tumor with low morbidity, sparing the patient of permanent stoma. However Tielen et al. (14) observed that 4 out of 12 patients who underwent local resection in their series developed local recurrence. In the present series, local excision was not feasible in any of the patients due to large size, locally advanced nature of the tumors or involvement of sphincters.
For those lesions which are not amenable for local excision, low anterior resection with coloanal anastomosis should be next best treatment option. Formal mesorectal excision is not necessary as lymphatic metastases are rare (3,15). Imatinib mesylate, a tyrosine kinase inhibitor which targets KIT and PDGFRA activated proteins, has become the standard therapy for advanced un-resectable/metastatic GISTs. In case of large sized or high-risk rectal GIST, which is not amenable to local excision or cannot be resected with free margins, preoperative therapy with imatinib may downsize the tumor, rendering the surgical procedure more conservative and much easier (16). Shrinkage of the tumor helps preserve function of the involved organs and may prevent intra-operative rupture of the tumor (17). Jakob et al. found that peri-operative imatinib therapy was associated with a higher rate of R0 resections and improved DFS and OS (18). In the present series, downsizing of the tumors was successful in nearly half of the patients with neoadjuvant therapy. However, this did not lead to a decrease in extent of surgical resection performed as APER was still required in 2/3rd patients. The tumor localization was the single most important factor preventing the performance of sphincter saving surgery in the present series. Tielen et al. also found that imatinib did not lead to less extensive surgery and the need for permanent stoma in their series was 40.6% (14). Possible hypothesis for this is that unlike rectal adenocarcinoma, response in cases of rectal GIST to imatinib therapy is usually metabolic rather than quantitative (no significant reduction in the size).
Optimal duration of neoadjuvant therapy remains controversial. General consensus is that surgical resection should occur at 6-12 months after start of imatinib. In the present series median duration of neoadjuvant therapy was 12 months. Scandinavian German adjuvant trial did show that optimum duration of adjuvant imatinib in high risk GIST patients is 3 years (6). According to Miettinen et al. rectal GISTs larger than 5 cm have a high risk of recurrence irrespective of mitotic count (19). In the present series all the patients who underwent surgical resection were planned for adjuvant imatinib for 3 years.
Given the long latency period between the primary surgical intervention and the recurrence and/or metastasis, regular follow-up at 3-6 months intervals in the first three postoperative years is highly recommended. In the present series only one patient developed distant metastasis. Wu et al. found that 3 and 5-year OS in their series of 61 patients of rectal GIST was 86% and 73.7% respectively (20). In the present series though median OS was not reached, the estimated 5-year survival was 90%. Considering the fact that unlike other series majority of the tumors in the present series were large in size and locally advanced, our results do suggest good prognosis for these tumors with R0 resection.
Limitations of the present series include small number of cases, shorter follow up and lack of mutational analysis in the great majority of the patients. The latter is significant since in the present era, adjuvant therapy with imatinib is frequently tailored according to the c KIT mutational analysis. However this is one of the few series with clear documentation of perioperative imatinib therapy.
Conclusions
Rectal GISTs offer special surgical challenge owing to the large size and proximity to the anal sphincter. Neoadjuvant imatinib may not improve sphincter preservation. Larger studies with longer follow up are required to establish evidence based recommendations for this rare yet biologically favorable malignancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Du CY, Shi YQ, Zhou Y, et al. The analysis of status and clinical implication of KIT and PDGFRA mutations in gastrointestinal stromal tumor (GIST). J Surg Oncol 2008;98:175-8. [PubMed]
- Kitamura Y, Hirotab S. Kit as a human oncogenic tyrosine kinase. Cell Mol Life Sci 2004;61:2924-31. [PubMed]
- Gervaz P, Huber O, Morel P. Surgical management of gastrointestinal stromal tumours. Br J Surg 2009;96:567-78. [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [PubMed]
- van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer 2005;104:1781-8. [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4.
- Centonze D, Pulvirenti E, Pulvirenti D'Urso A, et al. Local excision with adjuvant imatinib therapy for anorectal gastrointestinal stromal tumors. Tech Coloproctol 2013;17:571-4. [PubMed]
- Arezzo A, Verra M, Morino M. Transanal endoscopic microsurgery after neoadjuvant therapy for rectal GIST. Dig Liver Dis 2011;43:923-4. [PubMed]
- Gervaz P, Huber O, Bucher P, et al. Trans-sacral (Kraske) approach for gastrointestinal stromal tumour of the lower rectum: old procedure for a new disease. Colorectal Dis 2008;10:951-2. [PubMed]
- Hellan M, Maker VK. Transvaginal excision of a large rectal stromal tumor: an alternative. Am J Surg 2006;191:121-3. [PubMed]
- Tielen R, Verhoef C, van Coevorden F, et al. Surgical management of rectal gastrointestinal stromal tumors. J Surg Oncol 2013;107:320-3. [PubMed]
- Santos Fernandes Gd, Castro Cotti GC, Freitas D, et al. Downstaging of a rectal gastrointestinal stromal tumor by neoadjuvant imatinib therapy allowing for a conservative surgical approach. Clinics (Sao Paulo) 2009;64:819-21. [PubMed]
- Fujimoto Y, Akiyoshi T, Konishi T, et al. Laparoscopic sphincter-preserving surgery (intersphincteric resection) after neoadjuvant imatinib treatment for gastrointestinal stromal tumor (GIST) of the rectum. Int J Colorectal Dis 2014;29:111-6. [PubMed]
- Hohenberger P, Oladeji O, Licht A, et al. Neoadjuvant imatinib and organ preservation in locally advanced gastrointestinal stromaltumors (GIST). J Clin Oncol 2009; 26:abstr 10550.
- Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 2013;20:586-92. [PubMed]
- Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol 2011;104:865-73. [PubMed]
- Wu X, Jiang W, Zhang R, et al. Clinicopathological analysis of 61 patients with rectal gastrointestinal stromal tumors. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:335-9. [PubMed]


