The relationship between pathologic nodal disease and residual tumor viability after induction chemotherapy in patients with locally advanced esophageal adenocarcinoma receiving a tri-modality regimen
Introduction
Patients with adenocarcinoma (ACA) of the esophagus and gastro-esophageal junction (E/GEJ) often only come to clinical attention after the disease has invaded the full thickness of the esophageal wall, and symptomatic dysphagia has developed. With disease of this extent, lymph node metastases are quite common, and have been identified in greater than 50% of symptomatic patients (1). While this disease may be amenable to curative intent therapy, survival remains poor. By convention, we refer to these patients as having loco-regionally advanced (LRA) disease.
The optimal management of patients with LRA ACA of the E/GEJ is undefined. Surgery alone is associated with unacceptably high rates of local and distant failure, and poor overall survival (OS) (2). Multiple investigators have explored the impact of peri-operative chemotherapy and chemo-radiotherapy (CRT) in this setting (3-10). Most trials have employed pre-operative (or neo-adjuvant) therapy given the concern that patients will either be unfit for, or decline, adjuvant post-operative therapy after esophagectomy.
It appears that both single modality chemotherapy and concurrent CRT when given pre-operatively, can improve the survival of patients with this disease compared to surgery alone (11). While no definitive comparison between these two treatment approaches has been reported, the literature supporting pre-operative CRT is more robust (12), and this treatment paradigm can be considered a standard of care.
A recurring observation that has emerged from the pre-operative CRT trials has been that patients with a complete pathologic response to induction therapy appear to have relatively favorable outcomes, with reported 5-year OSs between 50-70% (13-16). This has led many to conclude that the key to a better outcome in this disease would be to use more aggressive induction regimens so as to increase the pathologic complete response rate. Less is known, however, about the prognostic significance of lesser pathologic responses, or about the importance of a complete or partial response after induction chemotherapy alone.
We previously reported the results of a phase II trial investigating the safety and efficacy of induction chemotherapy followed by surgery and adjuvant CRT (17). This trial was based on the observation that current multimodality treatments result in excellent loco-regional control, and that distant metastases represent the most common cause of treatment failure and death. Our protocol was designed to increase the delivery of potentially active systemic chemotherapy in hopes of reducing distant metastasis, while maintaining the loco-regional control provided by CRT, thereby improving OS. In our prior report, we described an association between pathologic tumor regression after induction chemotherapy, measured as residual tumor viability, and treatment outcomes (17). In this report, we have further evaluated the prognostic significance of residual viability (RV) and considered it within the context of pathologic nodal disease dissemination.
Methods
Study design
This is a post hoc exploratory investigation of a single arm phase II study. Results for the entire cohort have been previously published (16). In this analysis, outcomes for the subset of patients who underwent curative intent surgical resection are reported, and correlations between pathologic variables and survival outcomes were examined. Research support for the phase II trial was provided by Sanofi-Aventis. This clinical trial was approved and reviewed yearly by the Case Comprehensive Cancer Center Institutional Review Board. All patients signed written informed consent before entry on trial.
Patients
Eligibility for the phase II study required that patients be at least 18 years of age, and have a histologically confirmed diagnosis of ACA of the esophagus or GEJ. Patients with other histologic subtypes, including squamous cell carcinoma or adeno-squamous carcinoma, were not eligible. A clinical stage of T3-4 or N1 or M1a (AJCC 6th edition) as assessed by an endoscopic ultrasound (EUS) and fused positron emission tomography/computerized tomography (PET/CT) scan was required. Pathologic confirmation of nodal dissemination by EUS guided fine needle aspiration was not required. An Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, normal bone marrow function (neutrophil count >1,500/mm3, platelet count >100,000/mm3), serum creatinine <1.6 mg/dL, total bilirubin ≤1.5 mg/dL, ALT, AST, and alkaline phosphatase ≤3 times the institutional upper limit of normal, and adequate cardiopulmonary function (FEV1 ≥50% predicted, ejection fraction >50%) were also required.
Treatment
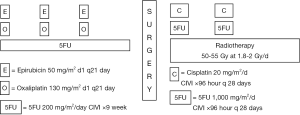
The treatment schema is illustrated in Figure 1. Patients received three cycles of induction chemotherapy with the EOF regimen which consisted of epirubicin (E) 50 mg/m2 IV on day 1, oxaliplatin (O) 130 mg/m2 IV on day 1, and 5FU (F) 200 mg/m2/day as a continuous intravenous infusion for 21 days. Cycles were repeated every 3 weeks. Approximately 3 weeks after completion of chemotherapy, patients were restaged with EUS and a CT of the chest, abdomen, and pelvis. All patients with loco-regionally confined disease, irrespective of whether there was any clinical evidence of loco-regional progression, then proceeded to surgery. Patients with new evidence of metastatic disease did not undergo resection but continued to be followed, and were treated at the discretion of the investigator.
Surgery was scheduled 4-5 weeks after the completion of chemotherapy. Patients underwent either a transthoracic esophagogastrectomy with a cervical esophagogastrostomy or a total gastrectomy with a Roux-en-Y esophagojejunostomy, usually through a left thoracoabdominal approach. An appropriate lymphadenectomy was also performed. A temporary feeding jejunostomy was placed in all patients for post-operative enteral nutritional supplementation.
Adjuvant CRT was initiated between 6 and 10 weeks post-operatively. External beam radiotherapy was delivered to the esophago-gastric tumor bed and draining lymphatic regions. For tumors of the GEJ and distal esophagus, at risk regional lymphatics included the lower mediastinum and celiac lymph nodes. For mid-esophageal tumors, all mediastinal lymph node stations were treated. The total dose of radiation was 50-55 Gy administered in 180-200 cGy daily fractions. Concurrent with radiotherapy, patients received 2 cycles of cisplatin and fluorouracil during the first and fourth weeks of treatment. Both agents were administered as continuous intravenous infusions over the course of 96 hours on a dedicated inpatient chemotherapy service. The total cisplatin dose per cycle was 80 mg/m2 administered at 20 mg/m2/day. The total 5FU dose per cycle was 4,000 mg/m2 administered at 1,000 mg/m2/day.
After the completion of treatment, patients were followed clinically every 8 to 12 weeks for the first three years. Follow-up beyond 3 years occurred at less frequent intervals. Recurrent disease was defined as loco-regional, distant, or both, and was histologically confirmed whenever possible.
Assessment of tumor down-staging and residual tumor viability
Pathologic response to induction chemotherapy was assessed in two ways: Tumor down-staging and residual tumor viability. Tumor down-staging was based on a comparison of the clinical stage determined by EUS prior to induction chemotherapy with the pathologic stage acquired after review of the surgical specimen. Complete tumor down-staging was defined by pT0N0 disease (which also corresponds with 0% residual tumor viability). Partial tumor down-staging was defined as a reduction in the pathologic T or N descriptor, without a reciprocal increase in the N or T descriptor, as compared to the initial clinical stage. Tumor upstaging was defined as any increase in the pathologic T or N descriptor. Stable disease reflected no change in the clinical and pathologic T and N descriptors.
Evaluation for residual tumor viability was based on the percentage of viable cancer cells in relation to surrounding fibrosis and acellular mucin pools in the tumor bed. The tumor bed identified on macroscopic examination was entirely submitted for histologic review in all cases. All pathology specimens were reviewed by a pathologist experienced in gastrointestinal oncology. RV was assessed prospectively using a quantitative scale at the time of the original pathologic evaluation of the surgical specimen.
Of note, tumor down-staging was defined according to the AJCC 6th edition, which was in use during the period of protocol design and initial participant enrollment. To further investigate the significance of nodal dissemination, however, pathologic staging was updated and reclassified according to the AJCC 7th edition (18). The AJCC 6th edition categorized pathologic celiac adenopathy as M1a disease for tumors of the distal esophagus and GEJ and defined any nodal disease as N1. The 7th edition does not distinguish M1a and M1b disease, and considers pathologic celiac nodal disease in the N descriptor. The 7th edition also better characterizes the extent of nodal dissemination, basing the N descriptor on the number of involved nodes.
Statistical considerations
Standard descriptive statistics were used to summarize patient and treatment characteristics. Outcomes were calculated from the date of surgery until the date of the event corresponding to each outcome, or the date of last follow-up. Outcomes of interest included distant metastatic control (DMC), defined by the event of recurrence in a distant site; recurrence-free survival (RFS) defined by the events of death from any cause, or any disease recurrence; and OS, defined by death from any cause. Recursive partitioning analysis (RPA) with a log-rank splitting method was used to identify optimal cut points in RV that best predict OS and RFS. RPA identified the same three groups relative to both outcomes, with low (0-25%), intermediate (26-75%), and high (>75%) RV. Study variables were compared among these three groups to see if patient or cancer characteristics are associated with RV. These tests were done using the Cochran-Armitage test (binary variables), Cochran-Mantel-Haenszel correlation test (ordinal variables), or Jonckheere-Terpstra test (continuous variables). Outcomes were estimated using the Kaplan-Meier method and compared by other study variables using the log-rank test. Stepwise Cox proportional hazards analysis with a variable entry criterion of P<0.10 and a variable retention criterion of P<0.05 was used to identify multivariable prognostic factors. Cox results were summarized as the hazard ratio (HR) and 95% confidence interval (CI) for HR. Analyses were conducted using SAS software (SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-sided, and P<0.05 was used to indicate statistical significance.
Results
Patient characteristics
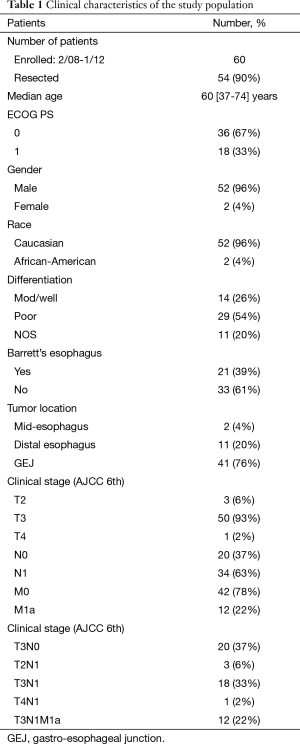
Between February 2008 and January 2012, 61 patients were enrolled on this protocol. One patient withdrew consent prior to receiving any treatment and is not included in this analysis, resulting in an eligible and evaluable cohort of 60 patients. Six patients were unable to undergo a curative intent resection. Pathologic review, therefore, was obtained in 54 patients. The clinical characteristics of these patients are detailed in Table 1. The vast majority were Caucasian males with T3 tumors of the distal esophagus and GEJ. Nodal dissemination was clinically suggested in 63% of patients.
Full table
Pathologic outcomes after induction chemotherapy
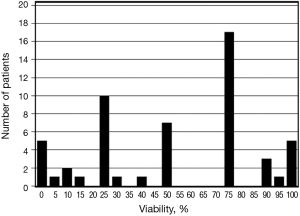
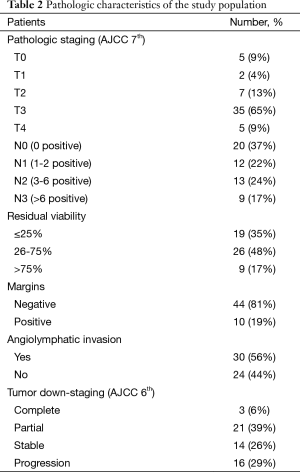
Table 2 describes the pathologic characteristics of these 54 patients. Tumor down-staging was achieved in 24 patients (46%). A complete pathologic response was identified in 3 patients (6%). Overall, 19 patients were reported to have ≤25% RV, 26 patients had 26-75% RV, and 9 patients had >75% RV. Thirty-four patients (63%) had residual histo-pathologic nodal dissemination, with similar numbers of patients with pathologic N1, N2, and N3 disease. A median of 32 (range, 7-69) lymph nodes were removed at surgery.
Full table
The reported RV is described as a frequency histogram in Figure 2. In univariate analysis, increasing RV was associated with pT3-4 tumors (P=0.016), increasing pN descriptor (P=0.014), the presence of extra-capsular nodal extension (P=0.025), and angio-lymphatic invasion (P<0.001). RV did not correlate with the presence of tumor down-staging (P=0.83).
Survival
With a median follow-up of 37 months, the Kaplan-Meier (KM) projected 3 year DMC, OS, and recurrence free survival of the 54 resected patients was 49%, 48%, and 44%, respectively. All three patients with a pathologic CR were alive and disease free at the time of this analysis, at 27, 35, and 42 months respectively. Outcomes were also defined by lesser degrees of pathologic regression, measured as RV (Figure 3A-C) and the pN descriptor (Figure 3D-F), in which DMC, OS, and RFS were progressively less favorable as the RV and number of pathologically involved lymph nodes increased. Tumor down-staging, however, was not associated with improved outcomes [HR OS 1.54 (0.71-3.34), P=0.28; HR RFS 1.50 (0.71-3.16), P=0.29].

In multivariable analysis (MVA), the RV and pN descriptor emerged as independent prognostic factors for RFS and OS (Table 3). Other variables included in multivariable analyses which were not of significant prognostic value for survival outcomes included the cT and cN descriptors, patient age, tumor length, angio-lymphatic invasion, tumor location (distal esophagus/GEJ), pathologic T descriptor, margin status (involved/uninvolved), and the presence of Barrett’s esophagus.
Full table
In patients with limited nodal disease (pN0-N1), recurrence free survival and OS could be further defined based on the extent of RV (Figure 4A,B). The 3-year OS of patients with low RV was 85%, compared to 51% in patients with intermediate or high viability (P=0.028). In contrast, RV did not appear to influence the outcomes of patients with more extensive nodal disease (Figure 4C,D). The 3-year OS of patients with pN2-N3 disease was 20% and 21% for patients with low RV and intermediate or high RV, respectively (P=0.55).
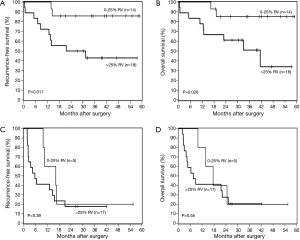
These observations suggest that the OS of patients treated in a similar fashion may be predicted by a combination of RV and the pN descriptor, and that three risk groups could be defined (low risk = pN0-1, <25% RV, intermediate risk = pN0-1, >25% RV, and high risk = pN2-3, any RV). The 3-year OS of low, intermediate, and high risk groups is 85%, 51%, and 20%, respectively (P=0.001).
Discussion
The administration of preoperative CRT often results in significant tumor regression. Common pathologic findings consistent with tumor regression include fibrosis and the presence of acellular mucin pools (19). Histologically, areas of fibrosis often co-exist in close proximity to clusters of viable cancer cells. Anatomically, regressive changes are not randomly distributed, and have been demonstrated to occur less frequently in the sub-mucosal and muscle layers of the esophagus (20). Histologic tumor regression has been associated with favorable outcomes and improved survival in several gastrointestinal malignancies. In esophageal cancer, this correlation has been limited to the observation that more than half of the patients who obtain a complete pathologic response to pre-operative CRT are alive at 5 years, which is significantly greater than what is observed in patients with less marked regression or in patients treated with surgery alone (14-16).
Much less, however, has been reported about the impact of pathologic regression in patients who receive chemotherapy alone. This point deserves attention. The impact of chemotherapy alone on primary site disease may be more reflective of the treatment effect on occult metastatic disease, the most common cause of treatment failure. It therefore may prove more predictive of OS than regression in the setting of concurrent CRT. In our patients, greater regression correlated with a lower likelihood of distant metastases, and better RFS and OS, thus confirming the original hypothesis behind the protocol design.
A complete pathologic response to chemotherapy is uncommon. The outcomes of these patients, however, appear to be excellent. In our study, at a median follow up of 37 months, the 3 patients (6%) who obtained a pathologic CR were all alive and free of disease. Similarly, Langer et al. reported the results of a study on 92 patients with esophageal ACA who received induction chemotherapy without radiation (21). While only 7 patients obtained a pathologic complete response, they were all alive with a minimum follow up of 6 years.
The prognostic value of lesser degrees of pathologic tumor regression has been less well described. It appears, however, that there may be a continuum of benefit associated with RV. In our study, we found that distinguishing patients with ≤25%, 25-75%, and >75% RV predicted DMC, RFS, and OS. In the study reported by Langer et al., for patients with residual disease, lesser amounts of tumor regression also had significant prognostic import, based on the percentage of viable tumor (median OS 51 months for 1-50% residual tumor, median OS 16 months for >50% residual tumor, P<0.001).
Assessment of tumor regression, however, remains problematic. Several methods of reporting regressive changes have been proposed (13). Intra and inter-observer variability when assessing tumor regression is not uncommon. It appears that most discordant findings occur when employing more complex 4 or 5 tier systems, which attempt to distinguish patients with varying degrees of limited residual disease (22). Our frequency histogram demonstrated that 80% of patients were identified as having either 0%, 25%, 50%, 75% or 100% viability. Only infrequently did our pathologists make more subtle distinctions. Karamitopoulou et al. suggested the use of a 3 tier system (22). Using this method, viability would be reported as 0% (pathologic complete response), 1-50%, and >50%. This distinction appears to provide high inter-observer concordance and improved prognostic value. Similar recommendations for a simplified 3 tier system have been made by other investigators (13).
Davies et al. recently reported the prognostic value of tumor regression and tumor down-staging in a large cohort of patients treated with various induction chemotherapy regimens in the United Kingdom (23). Similar to our study, down-staging was evaluated by comparing the initial clinical stage with the final pathologic stage. In this report, approximately 44% of patients who received induction therapy demonstrated evidence of tumor down-staging, and there was a strong correlation between tumor down-staging and tumor regression. Overall, any tumor down-staging was associated with improved 5-year OS (52.5% vs. 12.6%, P<0.001). On multivariate analysis, tumor down-staging, lympho-vascular invasion, and margin status were independent predictors of OS.
We also evaluated the impact of tumor down-staging, pathologic stage, and tumor regression (measured as RV) on survival outcomes, and our findings are consistent with those of Davies et al., and emphasize the significant prognostic value of the response to induction chemotherapy. In our study, however, only the pN descriptor and the tumor RV were independently able to predict RFS and OS. On multivariate analysis, tumor down-staging, the pT descriptor, angio-lymphatic invasion, and the margin status failed to emerge as significant independent prognostic factors. Of note, while RV correlated with the pathologic T and N descriptors on univariate analysis, it did not correlate with tumor down-staging in our study. We suggest, therefore, that RV and the final pathologic stage (in particular pN) may more accurately reflect the tumor response to induction chemotherapy and may be more sensitive predictors of distant recurrence and survival outcomes than tumor down-staging per se.
This was an exploratory analysis of a prospective phase II trial, evaluating just 54 patients and our results can only be considered hypothesis generating. If validated, how could we incorporate this information into clinical practice? At first glance, the utility of a pathologic prognostic marker appears limited when applied to the management of esophageal cancer, as it can be identified only after induction therapy and surgical resection. Continued adjuvant treatment remains challenging, given the delayed recovery typically associated with multi-modality therapy. We have, however, previously reported the feasibility of post-operative adjuvant CRT in this patient population (17,24). Several potential applications of these observations therefore appear worthy of exploration. For example, while local failure remains a common concern in this disease, CRT is associated with significant morbidity and the potential for long term sequelae (25-27). Perhaps radiotherapy, or adjuvant therapy altogether, could be omitted or significantly modified in patients with low risk pathology (N0-1, ≤25% viable). Furthermore, patients with high risk pathology (N2-N3, any RV) after induction chemotherapy may benefit from alternative adjuvant regimens or the inclusion of CRT.
In summary, our study is one of the few reports evaluating the impact of RV after induction chemotherapy in patients with LRA ACA of the E/GEJ, and suggests that RV and the pN descriptor are independent prognostic factors for RFS and OS. These findings are consistent with the results reported by other investigators treating patients in a similar fashion (21-23,28,29). The use of RV appears most valuable in patients with low nodal disease burden, distinguishing those patients with relatively favorable and unfavorable prognoses. In patients with more advanced nodal disease, the outcomes appear to be poor regardless of RV. These findings, however, will require further investigation and validation before they can be incorporated into clinical trial design or routine clinical practice.
Acknowledgements
Research support was provided by Sanofi-Aventis. Dr. Adelstein has received research funding from Sanofi-Aventis and GaxoSmithKline.
Footnote
Conflicts of Interest: Previous Presentation (Poster Presentation, ASCO Gastrointestinal Cancers Symposium, January 15-17, 2015, San Francisco, CA, USA).
References
- Feith M, Stein HJ, Siewert JR. Pattern of lymphatic spread of Barrett's cancer. World J Surg 2003;27:1052-7. [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [PubMed]
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [PubMed]
- Donohoe CL, O'Farrell NJ, Grant T, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg 2013;258:784-92; discussion 792. [PubMed]
- Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009;87:392-8; discussion 398-9. [PubMed]
- Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [PubMed]
- McNamara MJ, Adelstein DJ, Bodmann JW, et al. A phase II trial of induction epirubicin, oxaliplatin, and fluorouracil, followed by surgery and postoperative concurrent cisplatin and fluorouracil chemoradiotherapy in patients with locoregionally advanced adenocarcinoma of the esophagus and gastroesophageal junction. J Thorac Oncol 2014;9:1561-7. [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol 2013;3:262. [PubMed]
- Shapiro J, ten Kate FJ, van Hagen P, et al. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg 2013;258:678-88; discussion 688-9. [PubMed]
- Langer R, Ott K, Feith M, et al. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol 2009;22:1555-63. [PubMed]
- Karamitopoulou E, Thies S, Zlobec I, et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am J Surg Pathol 2014;38:1551-6. [PubMed]
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32:2983-90. [PubMed]
- Adelstein DJ, Rice TW, Rybicki LA, et al. Mature results from a phase II trial of postoperative concurrent chemoradiotherapy for poor prognosis cancer of the esophagus and gastroesophageal junction. J Thorac Oncol 2009;4:1264-9. [PubMed]
- Adelstein DJ, Rice TW, Rybicki LA, et al. Does paclitaxel improve the chemoradiotherapy of locoregionally advanced esophageal cancer? A nonrandomized comparison with fluorouracil-based therapy. J Clin Oncol 2000;18:2032-9. [PubMed]
- Murthy SC, Rozas MS, Adelstein DJ, et al. Induction chemoradiotherapy increases pleural and pericardial complications after esophagectomy for cancer. J Thorac Oncol 2009;4:395-403. [PubMed]
- Adelstein DJ, Rice TW, Becker M, et al. Use of concurrent chemotherapy, accelerated fractionation radiation, and surgery for patients with esophageal carcinoma. Cancer 1997;80:1011-20. [PubMed]
- Lorenzen S, Thuss-Patience P, Al-Batran SE, et al. Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol 2013;24:2068-73. [PubMed]
- Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg 2005;242:684-92. [PubMed]