Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality
Introduction
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is associated with improved survival for patients with abdominal malignancies with peritoneal dissemination (1-3), although it was initially viewed with skepticism as a highly morbid procedure. However, a large volume of mostly retrospective data suggests that CRS and HIPEC has rates of morbidity and mortality similar to other major operations for abdominal malignancies. Identifying patient and tumor characteristics associated with an increased risk of complications is important as serious postoperative complications can significantly impact on quality of life, may delay other treatments, and may be associated with early recurrence following CRS and HIPEC (4).
Overall morbidity and mortality
In several large series of CRS and HIPEC for a variety of cancer types, the rates of grade III-IV morbidity range from 22-34% and mortality from 0.8-4.1% (Table 1) (5-10). In series from large centers including primarily patients with peritoneal dissemination of colorectal cancer (CRC), pseudomyxoma peritonei (PMP), or diffuse malignant peritoneal mesothelioma (DMPM), mortality is generally in the range of 2-4% (5,7,11-13). The few existing large series of HIPEC for gastric or ovarian cancer (OC) suggest mortality rates may be somewhat higher in gastric cancer (3.9% and 6.5% in series of 152 and 159 patients, respectively) (14,15), and somewhat lower in OC (0.8% in one of the largest series) (8). The higher mortality observed with CRS and HIPEC for gastric cancer may be related to gastrectomy, while the lower mortality observed with CRS and HIPEC for OC may be due to fewer visceral resections on average than CRS and HIPEC for primary gastrointestinal cancers. Specific complication rates from select large series are shown in Table 2. Common major postoperative complications include neutropenia, digestive fistula, pneumonia, postoperative bleeding, intra-abdominal abscess, systemic sepsis, wound infection, and renal insufficiency.
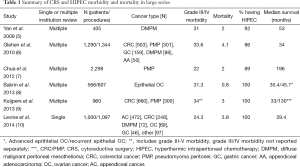
Full table
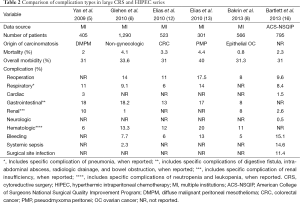
Full table
Specific patient and operative factors that have been examined for their contribution to CRS and HIPEC morbidity and mortality are discussed below. Those factors associated with CRS and HIPEC morbidity and mortality based on current evidence are summarized in Table 3.

Full table
Patient factors contributing to morbidity and mortality
Age
Morbidity and mortality from CRS and HIPEC is more common in elderly patients. Increasing age has been shown to be significantly associated with morbidity and mortality by univariate and multivariate analysis of HIPEC morbidity using large multi-institution series (6,17) and CRS and intraperitoneal chemotherapy data from the National Surgical Quality Improvement Program (NSQIP) database (16). In a single institution review of 81 patients over age 70 undergoing CRS and HIPEC, morbidity was comparable to other studies at 38%, while 30- and 90-day mortality were significantly higher at 13.6% and 27.4% (18). Analysis of age related morbidity and mortality from CRS and intraperitoneal chemotherapy using NSQIP data showed that age ≥60 years was independently associated with death and serious morbidity, which increased at a significant rate of 0.6% per year after age 50. Venous thromboembolism, sepsis, postoperative bleeding, and respiratory complications were more commonly seen complications in patients aged 60 years and older (19). In one small study of CRS and HIPEC following neoadjuvant chemotherapy for epithelial OC, morbidity was significantly greater in patients ≥75 years of age with no survival benefit in this group (20).
Hypoalbuminemia
Hypoalbuminemia has also been associated with morbidity and mortality from CRS and HIPEC in multiple studies. Lower preoperative serum albumin level was an independent predictor of 30-day mortality in one single institution study (21), while preoperative albumin <3 g/dL was associated with 58% morbidity and mortality rates on review of the NSQIP database (16).
Preoperative performance status
Poor preoperative performance status is an important predictor of morbidity and mortality following CRS and HIPEC. Ihemelandu et al. reported that a higher ECOG score is associated with increased 30-day morbidity (21), while Baratti et al. found that ECOG performance status >0 is an independent predictor of grade III-V morbidity (22).
Obesity
Obesity has not been consistently shown to be associated with a significant increase in overall HIPEC morbidity and mortality, although certain complications may be more common in obese patients. In a review of 1,000 patients having CRS and HIPEC procedures for primary tumors of the colon and appendix, 272 of whom were obese, neither 30-day major or minor morbidity, 30-day readmission rate, nor 30-day mortality (1.5% vs. 2.5%, obese vs. non-obese) were significantly associated with obesity. However, obese patients were more likely to have a late readmission, late urinary tract infection, and late anemia requiring blood transfusion (postoperative day 31-90). When analyzed by degree of obesity, moderately obese patients were more likely to have a late gastrointestinal bleed while severely obese patients were more likely to have a late exploratory laparotomy, intra-abdominal abscess, interventional radiology drain placement, urinary tract infection, anemia, and arrhythmia (23). In another single institution review of 114 HIPEC procedures, 22 in obese patients, overweight patients were more likely to have a deep vein thrombosis, but other complication rates were no different in obese compared to non-obese patients (24).
Operative factors affecting morbidity and mortality
Peritoneal carcinomatosis index (PCI)
The PCI, an indicator of extent of peritoneal disease, is one of the most consistent independent predictors of morbidity and/or mortality from CRS and HIPEC (6-8,17,22). One likely explanation for this finding may be that a higher PCI is a surrogate for more extensive surgery with the potential for more complications. Additionally, patients with more advanced disease may be more debilitated by their disease or have undergone more extensive preoperative therapy.
Bowel resection
Anastomotic leak and intestinal fistula are well known potential complications of CRS and HIPEC procedures. Multiple studies have shown that postoperative complications are more common when bowel anastomoses are required at the time of CRS and HIPEC (25,26). In a study of CRS and HIPEC for OC, the need for bowel resection was an independent predictor of morbidity (27). In a NSQIP analysis, gastrectomy with intraperitoneal chemotherapy, in particular, was associated with a high combined morbidity and mortality rate of 62% (16).
Diaphragmatic involvement
Diaphragmatic involvement has been associated with increased morbidity and mortality in CRS and HIPEC procedures. In a review of 1,077 procedures, 102 of which included diaphragmatic resection, major morbidity was similar with and without diaphragmatic resection (23.5% vs. 16.8%, P=0.10), but diaphragmatic resection increased 90-day mortality (12.8% vs. 6.12%, P=0.03) (28). In another study of 199 patients having CRS and HIPEC, 89 of whom had diaphragmatic involvement, diaphragmatic involvement increased 30-day major morbidity (29% vs. 15%, P=0.02), but did not affect 90-day mortality. In this study, patients with diaphragmatic involvement had longer operative times, greater transfusion requirements, less optimal cytoreduction, longer intensive care unit (ICU) stay, and longer hospital stays (29).
Distal pancreatectomy
Studies suggest that distal pancreatectomy at the time of CRS and HIPEC is safe but is also associated with increased morbidity and mortality. In a review of 118 CRS and HIPEC procedures at seven institutions that included distal pancreatectomy, the major complication rate and 90-day mortality rate were 44% and 7.6%, respectively, slightly higher than the anticipated rates of the procedure in general; the pancreatic fistula rate specifically was 33% (30). In another single institution study of 63 CRS and HIPEC procedures that included distal pancreatectomy out of 1,019 total procedures, distal pancreatectomy was not associated with increased mortality but was associated with increased major morbidity (30.2% vs. 18.8%, P=0.031) (31). Finally, a recent comparison of the perioperative pancreatic fistula (POPF) rate for distal pancreatectomy performed at the time of CRS and HIPEC for colorectal or appendiceal peritoneal carcinomatosis compared to the POPF rate for distal pancreatectomy for resectable pancreatic adenocarcinoma showed no difference in the POPF rate, although the rate of serious POPF (grade B or C) was significantly higher for distal pancreatectomy with CRS and HIPEC compared to distal pancreatectomy alone (32).
Hepatobiliary procedures
While there is a general consensus that hepatobiliary resections can be performed safely at the time of CRS and HIPEC, they may also be associated with an increase in morbidity and mortality. In a review of 252 CRS and HIPEC procedures with 63 involving hepatobiliary resection, the minor complication rate was 35%, the major complication rate was 33%, and the bile leak rate was 4.8%. The most common major complications were intra-abdominal abscess and pancreatitis (33). This major complication rate is similar to major complication rates for CRS and HIPEC procedures in general. In another study of patients having hepatic resection at the time of CRS and HIPEC, grade III/IV morbidity was similar with or without hepatic resection (18.9% vs. 22.5%, P=0.39), but there was a trend toward increased mortality (6.5% vs. 2.8%, P=0.07) in patients undergoing hepatic resection (34).
Urologic procedures
Results on the impact of urologic procedures to overall CRS and HIPEC morbidity are mixed. In one series of 267 patients having CRS and HIPEC for CRC with peritoneal carcinomatosis, 38 patients had an associated urologic procedure. The serious complication rate was 47% vs. 20% (P<0.001) for patients having a urologic procedure vs. no urologic procedure, although there was no difference in overall survival between groups. The most common complications were digestive fistula and intra-abdominal abscess (35). In three other series of 864, 598 and 170 patients having CRS and HIPEC with 7.3%, 8% and 20% including urologic procedures, respectively, urologic procedures did not increase major morbidity (36-38). In the former two of these studies, complications from urologic procedures were more common in malnourished patients, again demonstrating the impact of preoperative nutritional status on CRS and HIPEC morbidity. Two of the aforementioned studies found urologic procedures were associated with higher blood loss, operative time, and length of hospital stay (35,36), while a third found urologic procedures were not associated with increased transfusion requirement, operative time, length of ICU admission, or length of stay (38).
Iterative CRS and HIPEC
Small series on the morbidity and mortality of repeat CRS and HIPEC for recurrent or progressive disease suggests that major morbidity is not significantly greater after the second procedure compared to the first. In one study of 62 patients having a second CRS and HIPEC procedure, the overall morbidity and mortality rate was 48% after the second CRS and HIPEC compared to 32% after the first procedure with nine grade III/IV morbidities and two deaths following the second CRS and HIPEC (39). Another series of 79 patients having iterative CRS compared to 466 having primary CRS showed morbidity and mortality were no different at 41% vs. 42% (P=0.806), and 0 vs. 1.2% (P=0.600) (40). A third series of 30 patients having a second CRS and HIPEC showed rates of severe morbidity after the second procedure compared to the first procedure of 40% vs. 30% (P=0.37) (41). In a very recent study of outcomes following repeat CRS and HIPEC for 44 patients with DMPM, there were no mortalities and grade III-V morbidity was extremely low at 2.3%. The authors attributed this low incidence of complications to a combination of progression along the learning curve and fewer peritonectomies and visceral resections in an iterative procedure (42). The risk of complications from an iterative procedure, as is the case for any CRS and HIPEC procedure, is probably very individualized and will depend on a variety of factors including the number of visceral resections, the burden of postoperative adhesions, and the intraperitoneal chemotherapy agents used.
Location of small and large bowel adenocarcinoma
Limited studies suggest that while overall survival may differ, there is no difference in perioperative morbidity and mortality based on tumor location for adenocarcinomas of the small and large bowel. In a study of 440 patients having HIPEC with complete cytoreduction for peritoneal carcinomatosis of the colon, rectum, appendix (not including pseudomyxoma), and small bowel, there was no difference in morbidity or mortality between groups, although 5-year survival was superior in patients with an appendix primary (43). The efficacy of CRS and HIPEC for rectal cancer has been questioned due to the retroperitoneal location of the primary tumor, but Votanopoulos et al. found no difference in overall survival, morbidity, or mortality with CRS and HIPEC for peritoneal carcinomatosis from colon vs. rectal cancer, suggesting HIPEC for rectal cancer is safe in appropriately selected patients (44).
High grade histology
Some patients with appendiceal or colorectal cancer with peritoneal dissemination have higher grade, more aggressive tumors with a poorer overall prognosis. Few studies directly compare morbidity and mortality outcomes of CRS and HIPEC for low vs. high grade tumors. High grade is a negative prognostic factor for overall survival for mucinous appendiceal cancer, but long term survival can be achieved with optimal cytoreduction and HIPEC (11,45). However, survival is significantly worse for high grade tumors with lymph node involvement (46,47). Signet ring cell cancer is an aggressive form of CRC, and patients with colorectal signet cell carcinomatosis appear to have less favorable prognoses following CRS and HIPEC (48-51). When considering patients with aggressive tumors such as signet ring cell cancer or high grade mucinous appendiceal cancer with extensive lymph node involvement for CRS and HIPEC, the potential morbidity and mortality of the surgery must be weighed against the expected survival.
Perioperative systemic chemotherapy
Studies suggest that perioperative systemic chemotherapy does not appear to increase the major morbidity from CRS and HIPEC with the possible exception of preoperative bevacizumab, which has been associated with increased operative complications. In a series of 45 patients undergoing CRS and HIPEC for high grade appendiceal cancer by Turner et al., the 26 patients that had neoadjuvant chemotherapy with or without bevacizumab did not have an increased rate of major morbidity (52). Likewise, in a study of CRS and HIPEC for peritoneal carcinomatosis from CRC, Ceelen et al. found that neoadjuvant chemotherapy with bevacizumab improved overall survival and did not increase CRS and HIPEC mortality, overall complication rate, or anastomotic leakage rate (53). Eveno et al., on the other hand, found that in patients with disseminated CRC treated with neoadjuvant chemotherapy with or without bevacizumab followed by CRS and HIPEC, major morbidity was more common in the bevacizumab group (34% vs. 19%, P=0.020), while the mortality rate was 6.2% vs. 3.9% (P=0.12), and the gastrointestinal fistula rate was 18% vs. 10% (P=0.300) (54). In another study of 116 patients with DMPM, 60 patients received preoperative chemotherapy, 30 received postoperative chemotherapy, and 55 received perioperative platinum and pemetrexed. The authors found no increase in major complications with perioperative chemotherapy (55).
Surgeon and institution experience
In analyses of the impact of experience with CRS and HIPEC on surgical outcomes, there is a clear learning curve associated with improved CRS and HIPEC morbidity and mortality outcomes (56-58). One single institution comparison of outcomes from the first 70 CRS and HIPEC procedures to the next 70 CRS and HIPEC procedures demonstrated that severe morbidity decreased from 30% to 10% with reduced transfusion requirement, operative time, and length of ICU stay for the second group (59). Other studies suggest that approximately 140-180 procedures are needed to minimize severe morbidity (60-62). Importantly, the learning curve is not necessarily purely technical; improved patient selection likely contributes to the improved outcomes seen at experienced centers (56).
Complications of individual intraperitoneal chemotherapy regimens
Mitomycin C (MMC)
The most common toxicity of MMC is myelosuppression. A 28% rate of myelosuppression has been reported with single agent MMC intraperitoneal chemotherapy (63). The consequence of grade IV neutropenia is profound, as 66% of patients (4/6) with grade IV neutropenia died in one study (64). In a review of 127 CRS and HIPEC procedures with MMC for appendiceal cancer, female gender and MMC dose per body surface area (BSA) were independent predictors of severe neutropenia with MMC (65).
Platinums
There is some evidence that high dose intraperitoneal oxaliplatin can predispose patients to bleeding complications and mild hepatic toxicity, while high dose cisplatin can be nephrotoxic. The phase II CHIPOVAC trial, a study of CRS and HIPEC with 30 minutes of 460 mg/m2 oxaliplatin for advanced epithelial OC, was closed early due to the high rate of serious adverse events. The overall grade III morbidity rate was 29%, but 9/31 patients had 13 exploratory laparotomies for intra-abdominal bleeding following CRS and HIPEC (66). Elias et al. also reported a 50% rate of unexplained peritoneal hemorrhage at the two most hypotonic concentrations of oxaliplatin in an early pharmacokinetics study of increasingly hypotonic oxaliplatin solutions (67). Ceelen et al. found a 460 mg/m2 oxaliplatin dose to be safe for CRS and HIPEC in a series of 52 patients, 63% with CRC, with a major morbidity rate of 24% and no mortalities. They did, however, have one patient with multiple episodes of recurrent, unexplained intra-abdominal bleeding. In addition, they report evidence of persistent mild hepatic toxicity one month after surgery as evidenced by mild elevations in glutamyl transferase and alkaline phosphatase (68). In a series of phase I trials of CRS and HIPEC with cisplatin for primary peritoneal mesothelioma by Park et al., the major toxicity of cisplatin was renal toxicity at doses greater than the maximum tolerated dose (69).
MMC vs. platinums
MMC and platinum compounds are the most commonly used intraperitoneal chemotherapy agents. Data on the efficacy and toxicity of MMC compared to platinum compounds as the intraperitoneal chemotherapy agent for HIPEC are mixed and may depend largely on histology.
In one 2008 review of 103 consecutive CRS and HIPEC procedures at a Swedish university, fewer complications were seen in patients with PMP receiving MMC than in patients with other diagnoses receiving platinum compounds (42% vs. 71%, P<0.05) (70). A 2014 comparison of 39 patients receiving oxaliplatin and 56 patients receiving MMC for CRS and HIPEC found a tendency toward more extra-abdominal complications with MMC but similar rates of intra-abdominal complications; only the patients receiving MMC experienced neutropenia/leucopenia (71). In a matched pair analysis of 80 patients undergoing CRS and HIPEC with either oxaliplatin or MMC and doxorubicin, there was no difference in morbidity (42.5% vs. 37.5%, P=0.648) or mortality (2.5% vs. 0%) (72). These studies are summarized in Table 4. Interestingly, in another study of patients having splenectomy as part of CRS and HIPEC, grade III and IV platelet and neutrophil toxicity was more common with oxaliplatin than MMC; there was no difference in the rate of hematologic complications in patients that had CRS and HIPEC without splenectomy (73).

Full table
A recent study suggests that the efficacy of MMC or oxaliplatin as an intraperitoneal chemotherapy agent for patients with CRC peritoneal carcinomatosis may depend on the peritoneal surface disease severity score (PSDSS). In this multi-institution review of 539 patients with complete cytoreduction, median overall survival for patients receiving MMC vs. oxaliplatin was 32.7 vs. 31.4 months (P=0.925). However, when stratified by PSDSS score, median overall survival for PSDSS I/II patients was 54.3 vs. 28.2 months for those receiving MMC vs. oxaliplatin, while median overall survival for PSDSS III/IV patients was 19.4 vs. 30.4 months (P=0.427) for those receiving MMC vs. oxaliplatin (74). Other studies of CRS and HIPEC for malignant peritoneal mesothelioma suggest that platinum compounds are superior to MMC in terms of overall survival and may be associated with decreased transfusion requirement and shorter length of hospital stay (75,76).
Platinum compounds are also sometimes used in conjunction with MMC. In one study of 247 consecutive CRS and HIPEC procedures, grade III-V systemic toxicity was more common with higher cisplatin doses as well as cisplatin and doxorubicin, compared to cisplatin and MMC (77).
Irinotecan
Given the success of the intravenous regimen of 5-fluorouracil, oxaliplatin, and irinotecan (FOLFOXIRI) in the treatment of metastatic CRC (78), irinotecan has been studied as another intraperitoneal chemotherapy agent for HIPEC. In a two institution study of 146 patients receiving either oxaliplatin alone or oxaliplatin plus irinotecan, the overall morbidity rate was significantly lower with oxaliplatin alone (34.9% vs. 52.4%, P=0.05) with no difference in overall or relapse free survival (79). A single institution study of 20 patients receiving intraperitoneal oxaliplatin vs. 12 patients receiving intraperitoneal irinotecan for CRS and HIPEC for colorectal or appendiceal cancer found no difference in complications but did find a trend toward improved survival with oxaliplatin (80). Together these studies suggest that irinotecan may not be an ideal intraperitoneal chemotherapy agent.
Melphalan
Melphalan is another drug that has been used instead of the traditional intraperitoneal chemotherapy agents in select cases with some success and acceptable morbidity and mortality. In one series of 25 patients receiving intraperitoneal melphalan, the rate of grade III-IV morbidity was 23%, with nine patients experiencing neutropenia (81). Another series of 34 patients found a 26% grade III and 17% grade IV complication rate with intraperitoneal melphalan for CRS and HIPEC (82). Melphalan may be an appropriate second line chemotherapy agent for patients having a repeat CRS and HIPEC (42).
Independent contribution of intraperitoneal chemotherapy to HIPEC morbidity
The few existing studies comparing outcomes of surgery for abdominal malignancies with and without intraperitoneal chemotherapy suggest that intraperitoneal chemotherapy does not greatly increase morbidity and mortality over surgery alone. A 2011 study by Yang et al. is the only randomized controlled trial published to date evaluating outcomes for CRS with and without HIPEC. In this study of 68 patients with gastric cancer with peritoneal carcinomatosis, the serious adverse event rate for patients randomized to CRS alone was 11.7%, while the serious adverse event rate for those randomized to CRS plus HIPEC was 14.7% (P=0.839) (83). In a review of patients having colorectal resection for CRC using the NSQIP database, propensity matched morbidity (41% vs. 45%, P=0.34), mortality (1.1% vs. 2.5%, P=0.26), and length of stay (12 vs. 11 days, P=0.27) were no different with and without intraperitoneal chemotherapy (84). In a comparison of 54 patients undergoing CRS for recurrent OC, 22 patients who had surgery prior to 2008 had CRS alone, and 32 patients who had surgery from 2008 on had CRS plus HIPEC, with no difference in morbidity between groups (23% vs. 28%, P=0.453) (85). A small single institution case-control study of 62 consecutive patients having CRS for CRC with peritoneal carcinomatosis showed higher 30-day morbidity for patients having CRS and HIPEC vs. patients having CRS alone (28.6% vs. 9.4%, P=0.11), although this difference was not statistically significant (86). Finally, in the previously mentioned study comparing the POPF rate for distal pancreatectomy performed at the time of CRS plus HIPEC for colorectal or appendiceal carcinomatosis compared to the POPF rate for distal pancreatectomy without HIPEC for resectable pancreatic adenocarcinoma, there was no difference in the POPF rate, although the rate of serious POPF (grade B or C) was significantly higher in the former group (31).
Other factors affecting morbidity and mortality
Several other factors have been shown in a handful of studies to be associated with increased morbidity and/or mortality. In a NSQIP analysis, increased operative time and intraoperative transfusion requirement were associated with increased morbidity and mortality (16). In individual large single or multi-institution studies, factors that have been associated with increased morbidity include longer operative time, ovarian origin of tumor, and presence of ascites (17); more than five visceral resections (22); prior operations (7); and the institution where HIPEC was performed (6). Finally, in one analysis of independent predictors of HIPEC morbidity and mortality, major morbidity was 100% in 9/426 patients with PCI >30, more than five visceral resections, and poor performance status (22). This finding stresses the importance of preoperative consideration of all factors contributing to CRS and HIPEC morbidity and mortality.
Conclusions
CRS and HIPEC for disseminated intra-abdominal malignancies is a complex procedure with the potential for high morbidity and mortality. However, when CRS and HIPEC is performed at experienced high volume centers, it can be associated with long term survival with acceptable morbidity and mortality rates.
The decision to perform CRS and HIPEC should be made in a multidisciplinary setting with consideration of the risks and benefits, the timing in relation to systemic chemotherapy, and the patient and operative factors associated with increased morbidity and mortality. Morbidity and mortality vary by patient factors such as age, performance status, and nutrition status, and operative factors such as PCI, the organ systems affected by disease, tumor histology, and surgeon experience. A better understanding of the patient and operative factors associated with morbidity and mortality allows for more informed patient selection and decision making.
While abundant high level data is lacking, the limited existing data suggest that the independent contribution of the intraperitoneal chemotherapy to overall morbidity is small, and that the majority of morbidity is related to the abdominal surgery itself. Larger studies evaluating the individual contribution of intraperitoneal chemotherapy to CRS and HIPEC morbidity and mortality and to long-term outcomes are needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5. [PubMed]
- Simkens GA, van Oudheusden TR, Luyer MD, et al. Serious postoperative complications affect early recurrence after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis. Ann Surg Oncol 2015;22:2656-62. [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [PubMed]
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [PubMed]
- Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 2013;39:1435-43. [PubMed]
- Kuijpers AM, Mirck B, Aalbers AG, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol 2013;20:4224-30. [PubMed]
- Levine EA, Stewart JH 4th, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg 2014;218:573-85. [PubMed]
- Votanopoulos KI, Russell G, Randle RW, et al. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol 2015;22:1274-9. [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [PubMed]
- Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 2010;36:456-62. [PubMed]
- Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol 2014;21:1147-52. [PubMed]
- Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370-7. [PubMed]
- Bartlett EK, Meise C, Roses RE, et al. Morbidity and mortality of cytoreduction with intraperitoneal chemotherapy: outcomes from the ACS NSQIP database. Ann Surg Oncol 2014;21:1494-500. [PubMed]
- Macrì A, Arcoraci V, Belgrano V, et al. Short-term outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: preliminary analysis of a multicentre study. Anticancer Res 2014;34:5689-93. [PubMed]
- Votanopoulos KI, Newman NA, Russell G, et al. Outcomes of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients older than 70 years; survival benefit at considerable morbidity and mortality. Ann Surg Oncol 2013;20:3497-503. [PubMed]
- Peters MG, Bartlett EK, Roses RE, et al. Age-related morbidity and mortality with cytoreductive surgery. Ann Surg Oncol 2015. [Epub ahead of print]. [PubMed]
- Cascales-Campos P, Gil J, Gil E, et al. Cytoreduction and HIPEC after neoadjuvant chemotherapy in stage IIIC-IV ovarian cancer. Critical analysis in elderly patients. Eur J Obstet Gynecol Reprod Biol 2014;179:88-93. [PubMed]
- Ihemelandu CU, McQuellon R, Shen P, et al. Predicting postoperative morbidity following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CS+HIPEC) with preoperative FACT-C (Functional Assessment of Cancer Therapy) and patient-rated performance status. Ann Surg Oncol 2013;20:3519-26. [PubMed]
- Baratti D, Kusamura S, Mingrone E, et al. Identification of a subgroup of patients at highest risk for complications after surgical cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg 2012;256:334-41. [PubMed]
- Votanopoulos KI, Swords DS, Swett KR, et al. Obesity and peritoneal surface disease: outcomes after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colon primary tumors. Ann Surg Oncol 2013;20:3899-904. [PubMed]
- McPartland SJ, Goodman MD. The effect of elevated body mass index on outcomes following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2014;21:1463-7. [PubMed]
- Verwaal VJ, van Tinteren H, Ruth SV, et al. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol 2004;85:61-7. [PubMed]
- Franko J, Gusani NJ, Holtzman MP, et al. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol 2008;15:3065-72. [PubMed]
- Cascales Campos P, Gil J, Parrilla P. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with primary and recurrent advanced ovarian cancer. Eur J Surg Oncol 2014;40:970-5. [PubMed]
- Ahmed S, Levine EA, Randle RW, et al. Significance of diaphragmatic resections and thoracic chemoperfusion on outcomes of peritoneal surface disease treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2014;21:4226-31. [PubMed]
- Franssen B, Tabrizian P, Weinberg A, et al. Outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on patients with diaphragmatic involvement. Ann Surg Oncol 2015;22:1639-44. [PubMed]
- Schwarz L, Votanopoulos K, Morris D, et al. Is the combination of distal pancreatectomy and cytoreductive surgery with HIPEC reasonable? Results of an international multicener study. Ann Surg 2015. [Epub ahead of print].
- Doud AN, Randle RW, Clark CJ, et al. Impact of distal pancreatectomy on outcomes of peritoneal surface disease treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2015;22:1645-50. [PubMed]
- Downs-Canner S, Ding Y, Magge DR, et al. A comparative analysis of postoperative pancreatic fistulas after surgery with and without hyperthermic intraperitoneal chemoperfusion. Ann Surg Oncol 2015;22:1651-7. [PubMed]
- Glockzin G, Renner P, Popp FC, et al. Hepatobiliary procedures in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2011;18:1052-9. [PubMed]
- Randle RW, Doud AN, Levine EA, et al. Peritoneal surface disease with synchronous hepatic involvement treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2015;22:1634-8. [PubMed]
- Braam HJ, van Oudheusden TR, de Hingh IH, et al. Urological procedures in patients with peritoneal carcinomatosis of colorectal cancer treated with HIPEC: morbidity and survival analysis. Anticancer Res 2015;35:295-300. [PubMed]
- Votanopoulos KI, Randle RW, Craven B, et al. Significance of urinary tract involvement in patients treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2014;21:868-74. [PubMed]
- Honoré C, Souadka A, Goéré D, et al. HIPEC for peritoneal carcinomatosis: does an associated urologic procedure increase morbidity? Ann Surg Oncol 2012;19:104-9. [PubMed]
- Leapman MS, Jibara G, Tabrizian P, et al. Genitourinary resection at the time of cytoreductive surgery and heated intraperitoneal chemotherapy for peritoneal carcinomatosis is not associated with increased morbidity or worsened oncologic outcomes: a case-matched study. Ann Surg Oncol 2014;21:1153-8. [PubMed]
- Votanopoulos KI, Ihemelandu C, Shen P, et al. Outcomes of repeat cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal surface malignancy. J Am Coll Surg 2012;215:412-7. [PubMed]
- Chua TC, Quinn LE, Zhao J, et al. Iterative cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent peritoneal metastases. J Surg Oncol 2013;108:81-8. [PubMed]
- Golse N, Bakrin N, Passot G, et al. Iterative procedures combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal recurrence: postoperative and long-term results. J Surg Oncol 2012;106:197-203. [PubMed]
- Ihemelandu C, Bijelic L, Sugarbaker PH. Iterative cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent or progressive diffuse malignant peritoneal mesothelioma: clinicopathologic characteristics and survival outcome. Ann Surg Oncol 2015;22:1680-5. [PubMed]
- Elias D, Glehen O, Pocard M, et al. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann Surg 2010;251:896-901. [PubMed]
- Votanopoulos KI, Swett K, Blackham AU, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from rectal cancer. Ann Surg Oncol 2013;20:1088-92. [PubMed]
- Jimenez W, Sardi A, Nieroda C, et al. Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2014;21:4218-25. [PubMed]
- Baumgartner JM, Tobin L, Heavey SF, et al. Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2015;22:1716-21. [PubMed]
- Halabi HE, Gushchin V, Francis J, et al. Prognostic significance of lymph node metastases in patients with high-grade appendiceal cancer. Ann Surg Oncol 2012;19:122-5. [PubMed]
- Chua TC, Pelz JO, Kerscher A, et al. Critical analysis of 33 patients with peritoneal carcinomatosis secondary to colorectal and appendiceal signet ring cell carcinoma. Ann Surg Oncol 2009;16:2765-70. [PubMed]
- Van Sweringen HL, Hanseman DJ, Ahmad SA, et al. Predictors of survival in patients with high-grade peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Surgery 2012;152:617-24; discussion 624-5. [PubMed]
- van Oudheusden TR, Braam HJ, Nienhuijs SW, et al. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J Surg Oncol 2015;111:237-42. [PubMed]
- Winer J, Zenati M, Ramalingam L, et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014;21:1456-62. [PubMed]
- Turner KM, Hanna NN, Zhu Y, et al. Assessment of neoadjuvant chemotherapy on operative parameters and outcome in patients with peritoneal dissemination from high-grade appendiceal cancer. Ann Surg Oncol 2013;20:1068-73. [PubMed]
- Ceelen W, Van Nieuwenhove Y, Putte DV, et al. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann Surg Oncol 2014;21:3023-8. [PubMed]
- Eveno C, Passot G, Goéré D, et al. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014;21:1792-800. [PubMed]
- Deraco M, Baratti D, Hutanu I, et al. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2013;20:1093-100. [PubMed]
- Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg 2007;94:1408-14. [PubMed]
- Turrini O, Lambaudie E, Faucher M, et al. Initial experience with hyperthermic intraperitoneal chemotherapy. Arch Surg 2012;147:919-23. [PubMed]
- Chua TC, Yan TD, Smigielski ME, et al. Long-term survival in patients with pseudomyxoma peritonei treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy: 10 years of experience from a single institution. Ann Surg Oncol 2009;16:1903-11. [PubMed]
- Yan TD, Links M, Fransi S, et al. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal surface malignancy--a journey to becoming a nationally funded peritonectomy center. Ann Surg Oncol 2007;14:2270-80. [PubMed]
- Kusamura S, Baratti D, Hutanu I, et al. The importance of the learning curve and surveillance of surgical performance in peritoneal surface malignancy programs. Surg Oncol Clin N Am 2012;21:559-76. [PubMed]
- Kusamura S, Baratti D, Deraco M. Multidimensional analysis of the learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann Surg 2012;255:348-56. [PubMed]
- Polanco PM, Ding Y, Knox JM, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol 2015;22:1673-9. [PubMed]
- Sugarbaker PH, Alderman R, Edwards G, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol 2006;13:635-44. [PubMed]
- Schnake KJ, Sugarbaker PH, Yoo D. Neutropenia following perioperative intraperitoneal chemotherapy. Tumori 1999;85:41-6. [PubMed]
- Lambert LA, Armstrong TS, Lee JJ, et al. Incidence, risk factors, and impact of severe neutropenia after hyperthermic intraperitoneal mitomycin C. Ann Surg Oncol 2009;16:2181-7. [PubMed]
- Pomel C, Ferron G, Lorimier G, et al. Hyperthermic intra-peritoneal chemotherapy using oxaliplatin as consolidation therapy for advanced epithelial ovarian carcinoma. Results of a phase II prospective multicentre trial. CHIPOVAC study. Eur J Surg Oncol 2010;36:589-93. [PubMed]
- Elias D, El Otmany A, Bonnay M, et al. Human pharmacokinetic study of heated intraperitoneal oxaliplatin in increasingly hypotonic solutions after complete resection of peritoneal carcinomatosis. Oncology 2002;63:346-52. [PubMed]
- Ceelen WP, Peeters M, Houtmeyers P, et al. Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann Surg Oncol 2008;15:535-41. [PubMed]
- Park BJ, Alexander HR, Libutti SK, et al. Treatment of primary peritoneal mesothelioma by continuous hyperthermic peritoneal perfusion (CHPP). Ann Surg Oncol 1999;6:582-90. [PubMed]
- van Leeuwen BL, Graf W, Pahlman L, et al. Swedish experience with peritonectomy and HIPEC. HIPEC in peritoneal carcinomatosis. Ann Surg Oncol 2008;15:745-53. [PubMed]
- Hompes D, D’Hoore A, Wolthuis A, et al. The use of oxaliplatin or mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol 2014;109:527-32. [PubMed]
- Glockzin G, von Breitenbuch P, Schlitt HJ, et al. Treatment-related morbidity and toxicity of CRS and oxaliplatin-based HIPEC compared to a mitomycin and doxorubicin-based HIPEC protocol in patients with peritoneal carcinomatosis: a matched-pair analysis. J Surg Oncol 2013;107:574-8. [PubMed]
- Votanopoulos K, Ihemelandu C, Shen P, et al. A comparison of hematologic toxicity profiles after heated intraperitoneal chemotherapy with oxaliplatin and mitomycin C. J Surg Res 2013;179:e133-9. [PubMed]
- Prada-Villaverde A, Esquivel J, Lowy AM, et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with mitomycin C versus oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol 2014;110:779-85. [PubMed]
- Blackham AU, Shen P, Stewart JH, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Ann Surg Oncol 2010;17:2720-7. [PubMed]
- Shetty SJ, Bathla L, Govindarajan V, et al. Comparison of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy with mitomycin or carboplatin for diffuse malignant peritoneal mesothelioma. Am Surg 2014;80:348-52. [PubMed]
- Kusamura S, Baratti D, Younan R, et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol 2007;14:2550-8. [PubMed]
- Masi G, Cupini S, Marcucci L, et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol 2006;13:58-65. [PubMed]
- Quenet F, Goéré D, Mehta SS, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg 2011;254:294-301. [PubMed]
- Glockzin G, Gerken M, Lang SA, et al. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: a retrospective analysis. BMC Cancer 2014;14:807. [PubMed]
- Sardi A, Jimenez W, Nieroda C, et al. Melphalan: a promising agent in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2014;21:908-14. [PubMed]
- Bijelic L, Sugarbaker PH, Stuart OA. Hyperthermic intraperitoneal chemotherapy with melphalan: a summary of clinical and pharmacological data in 34 patients. Gastroenterol Res Pract 2012;2012:827534.
- Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. [PubMed]
- Bartlett EK, Choudhury RA, Roses RE, et al. Intraperitoneal chemotherapy at the time of surgery is not associated with increased 30-day morbidity and mortality following colorectal resection. Ann Surg Oncol 2015;22:1664-72. [PubMed]
- Cascales-Campos PA, Gil J, Feliciangeli E, et al. The role of hyperthermic intraperitoneal chemotherapy using paclitaxel in platinum-sensitive recurrent epithelial ovarian cancer patients with microscopic residual disease after cytoreduction. Ann Surg Oncol 2015;22:987-93. [PubMed]
- Huang CQ, Feng JP, Yang XJ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol 2014;109:730-9. [PubMed]


