
Combination of preoperative red cell distribution width and neutrophil to lymphocyte ratio as a prognostic marker for gastric cancer patients
Introduction
Gastric cancer (GC) is the second leading of cause of cancer-related death in China (1) and places a large economic burden on society according to the latest epidemiologic data (2). Multimodality treatment approaches, consisting of surgery, adjuvant chemotherapy, immunity therapy, and targeted therapy, have improved patient outcomes in GC patients in recent decades, and radical surgery with a standard D2 lymphadenectomy remains the prioritized choice for stage II–III GC patients (3), when supplemented by chemotherapy and/or molecular targeted drugs (4). However, due to the heterogeneity of the treatment response to these therapies in most advanced GC patients, the prognosis remains unsatisfactory because of systemic relapses after surgery. Therefore, the discovery of a novel reliable biomarker to predict tumor recurrence or metastasis is imperative and will help risk stratification and improve prognostication in stage II–III GC patients.
The past decade has seen a growing consensus that cancer-related immune-inflammatory responses are critically involved in tumor progression by promoting tumor growth, invasion, angiogenesis, immune escape, chemoresistance, and metastasis, and are associated with the prognosis of the cancer patients (5-7). Recently, immune-inflammatory markers have been reported to be correlated with the prognosis of GC patients, including the circulating neutrophil to lymphocyte ratio (NLR) (8). Red blood cell distribution width (RDW) has obtained increasing attention in cancer research and several studies have reported that its elevation also correlates with a poor prognosis in GC (9). RDW is composed of RDW-variation coefficient (RDW-cv) and RDW-standard deviation (RDW-sd), and reflects the evenness of the volume and size of red blood cells (RBCs) (10). RDW is a component of routine blood examinations, and several studied reported that its elevation was associated with poor nutritional status and cancer-related chronic inflammation (11).
However, the combined score of RDW and NLR in the prognostic value of stage II–III GC patients remains unclear. Therefore, we proposed that a cumulative score based on preoperative RDW-cv and NLR (R-NLR score) might provide more accuracy in predicting the long-term survival of stage II–III GC patients who receive radical surgery. Based on this, the purpose of this study was to examine the correlation of the R-NLR score with clinicopathologic variables, and to investigate its prognostic significance in stage II–III GC patients as a mechanism for guiding clinical therapy to improve the survival of these patients.
We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-271).
Methods
Patients
The electronic medical records of 151 patients diagnosed with stage II–III GC between March 2014 and September 2015 and hospitalized in The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital were retrospectively reviewed (Figure S1). All enrolled GC patients were treated with radical surgery and had complete follow-up. The inclusion criteria for selected patients were as follows: (I) all GC patients were confirmed by biopsy; (II) GC was defined according to pathological diagnosis and primary tumors were staged by CT; (III) all patients received radical gastrectomy and D2 lymphadenectomy; (IV) there was no evidence of distant metastasis; and (V), six to eight cycles of postoperative chemotherapy using 5-fluorouracil (5-FU)-based regimens (mostly oxaliplatin with either S-1) were performed after surgery. The exclusion criteria were any of the following: (I) patients with infection, rheumatoid disease or other inflammatory conditions, blood transfusion, or hematopoietic cytokine [i.e., epidermal growth factor (EGF), granulocyte colony-stimulating factor (G-CSF) within 1 month of study onset]; (II) evidence of distant metastasis at the first record of hospitalization; (III) remnant GC; (IV) patients who underwent non-radical surgery, or died due to non-cancer-related causes; (V) patients who underwent emergency surgery performed in case of digestive bleeding or perforation; and (VI), those who did not complete postoperative chemotherapy and follow up.
Clinical and laboratory variables
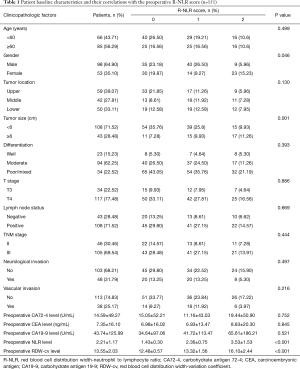
Variables including age, sex, tumor size, pathology, T stage, N stage, TNM stage, metastatic lymph node, vascular invasion, neurological invasion, serum carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 72-4 (CA72-4), preoperative neutrophil, lymphocyte, and pre-RDW-cv levels were collected from the first record of hospitalization for each patient. Tumor stages were classified according to the AJCC TNM staging system (8th edition) (12) and NLR was defined as the ratio of the neutrophil count over the lymphocyte count. All patients were divided into a high and low group according to the optimal cut-off points of RDW-cv and NLR by SPSS 24.0 software. The optimal cut-off value for preoperative- RDW-cv was defined as 14.1% and the cut-off value for pre-NLR was determined as 2.015. Patients with elevated RDW-cv (≥14.1%) and elevated pre-NLR (≥2.015) levels were allocated an R-NLR score of 2, an R-NLR of 1 (either a high RDW-cv or a high NLR), and R-NLR score of 0 (neither a high RDW-cv nor a high NLR). This study was approved by the Ethics Committee of The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital (No. KY2019177). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from participating patients.
Follow-up
Patients were followed at 3-month intervals for the first 2 years and at 6-month intervals for the next 3 years, and the median follow-up was 52.01 (range, 18–64) months after surgery. Patients who did not visit our hospital as scheduled were telephoned for follow-up to obtain treatment information and living status. Recurrence was determined by clinical and radiologic examination and/or histologic confirmation, including gastroscopy, serum CEA, CA19-9, CA72-4, CT, MRI, and/or PET-CT, and examinations were performed once metastasis was suspected. Overall survival (OS) was defined as the time from the diagnosis of GC to the date of the last follow-up or death, and disease-free survival (DFS) was defined as the time from surgery to the time of relapse.
Statistical analysis
The data were analyzed by SPSS 24.0 software (IBM SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation if normally distributed and categorical data were presented as numbers (percentage). Survival analysis was calculated by the Kaplan-Meier method and differences between the survival curves were compared with the log-rank test. Univariate and multivariate analyses were performed by Cox proportional hazards regression models, and risk factors of DFS and OS were calculated by hazard ratios (HRs). HRs with 95% confidence intervals (CIs) and two-sided P values were analyzed. A two-sided P<0.05 was considered statistically significant.
Results
Clinical characteristics of patients
A total of 151 patients were eligible for the analysis, including 98 males and 53 females with an average age 56.65±9.85 (range, 28–79) years. Most patients (77.48%) presented with T4 disease, and lymph node metastasis was positive in 108 (71.52%), with 46 (30.46%) having stage II and 105 (69.54%) having stage III disease. The average preoperative NLR and RDW-cv levels were 2.21±1.17 and 13.55±2.03, respectively.
Prognostic value of preoperative RDW-cv and NLR in advanced GC patients
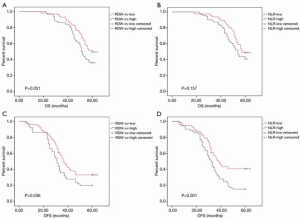
The 5-year OS rates in low and high RDW-cv groups were 49.5% and 36% (P=0.051, Figure 1A), while in the low and high NLR groups these were 48.8% and 40.8%, respectively (P=0.157, Figure 1B). Interestingly, the 5-year DFS rate was significantly improved in low RDW-cv patients compared with those with high RDW-cv (33.7% vs. 20.0%, P=0.036, Figure 1C) and was significantly lower in high NLR patients than in low NLR patients (15.5% vs. 41.2%, P=0.001, Figure 1D).
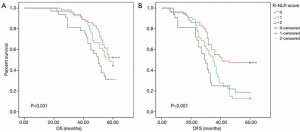
Prognostic significance of preoperative R-NLR score in advanced GC patients
After a median follow-up of 52.01 (range, 18–64) months, a survival analysis was then performed to evaluate the prognostic value of the preoperative R-NLR score. According to the criteria of the R-NLR score, 65 (43.05%), 54 (35.76%), and 32 (21.19%) of patients had an R-NLR score of 0, 1 and 2, respectively. The preoperative RDW-cv levels in the R-NLR 0, 1, and 2 groups were 12.48±0.57, 13.32±1.56, and 16.10±2.44 (P<0.001), respectively, while the preoperative NLR level in the R-NLR 0, 1, and 2 groups was 1.43±0.30, 2.36±0.75, and 3.53±1.53 (P<0.001), respectively. As shown in Table 1, an elevated R-NLR score was associated with bigger tumor size (P=0.001). The 5-year OS in the R-NLR 0, 1, and 2 groups was 52.30%, 44.40%, and 31.20% (P=0.031), respectively, and the 5-year DFS in the R-NLR 0, 1, and 2 groups was 47.70%, 18.80%, and 13.00% (P<0.001), respectively (Figure 2).
Full table
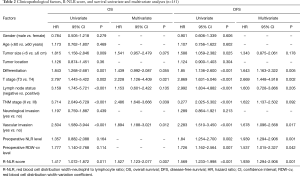
A Cox univariate model for OS and DFS was utilized to evaluate the prognostic value of the preoperative R-NLR score, and the results revealed that a high preoperative R-NLR score was significantly associated with poor OS (HR, 1.527; 95% CI, 1.123–2.077; P=0.007) (Figure 2A). Simultaneously, vascular invasion (HR, 1.894; 95% CI, 1.188–3.021; P=0.012), depth of invasion (HR, 2.228; 95% CI, 1.126–4.409; P=0.021), and TNM stage (HR, 2.486; 95% CI, 1.640–3.666; P=0.039) were other significant prognostic parameters identified by multivariate analysis, while the preoperative R-NLR score (HR, 1.939; 95% CI, 1.294–2.906; P=0.001) remained an independent prognostic indicator for DFS. Similarly, tumor differentiation (HR, 1.643; 95% CI, 1.163–2.322; P=0.005), depth of invasion (HR, 2.669; 95% CI, 1.448–4.918; P=0.021), vascular invasion (HR, 1.678; 95% CI, 1.096–2.568; P=0.017), preoperative NLR level (HR, 1.939; 95% CI, 1.294–2.906; P=0.001), and preoperative RDW-cv level (HR, 1.537; 95% CI, 1.015–2.327; P=0.042) were other independent prognostic factors for DFS (Table 2).
Full table
Discussion
A number of previous studies have compared the prognostic impact of SII, CRP and other inflammation-based parameters in tumors such as lung and colon cancers (13-16). However, to our knowledge, there are no studies on the most appropriate parameters to predict long-term prognosis in GC. Some inflammatory and nutritional markers, including NLR and RDW, have been shown to indirectly reflect the progression of GC (13,14). Malnutrition is one of the most common symptoms of GC, and the use of preoperative anemia as a nutritional indicator is easy and convenient. The most common cause of anemia is iron deficiency caused by cancer progression, which leads to a serious decline in the physical condition of patients with GC (15). RDW is obtaining increasing attention in patients with GC, and its elevation has been reported to be correlated with a poor prognosis (16). Recent research also demonstrated that elevated RDW was associated with systemic inflammation induced by cancer cells and the cancer microenvironment (17-19). Increased cancer-related inflammation may inhibit the production of erythropoietin, reduce the release of iron from reticuloendothelial macrophages, and shorten the survival time of RBCs as cancer progresses (20). Interestingly, elevated RDW reflects these changes and is indirectly related to cancer-related inflammation, although the underlying mechanism requires further research.
Cancer-related inflammation appears to play a vital role in the development of several cancers including GC. Therefore, “avoiding immune destruction” has been accepted as an emerging hallmark and contributing characteristic of cancer treatment, including that of GC (21,22). Simultaneously, increasing evidence has shown that several inflammation indicators such as NLR are associated with tumor recurrence and a worse prognosis in several cancers (23-25). However, few reports have studied the relationship between NLR and the prognosis of patients with stage II–III GC. More importantly, there is now enough evidence to suggest that RDW and NLR are related to the prognosis of patients with stage II–III GC. However, there are no previous studies investigating the combined analysis of perioperative RDW and NLR (R-NLR score) for the prognostic estimation in stage II–III GC patients with D2 lymphadenectomy.
In the present study, we demonstrated that RDW-cv and NLR correlated with the prognosis of GC by AUC, despite RDW-cv being rarely reported as doing so. NLR is considered an important prognostic marker which reflects the tumor-related inflammatory state in several cancers including GC (26). However, our results suggest that elevated RDW-cv and NLR are negatively correlated with DFS but not OS in stage II–III GC patients who received radical surgery. Rapid economic growth in China has facilitated the development of secondary surgery and targeted drugs therapy applications following tumor recurrence and metastasis (27). However, the reliance on single tumor markers to assess the prognosis of cancers such as GC retards this progress. In this study, we demonstrated that the R-NLR score, which is based on RDW and NLR, could increase the predictive prognostic accuracy of stage II–III GC patients. We found that GC patients with elevated R-NLR have a worse 5-year OS and shorter DFS compared to those with low R-NLR. In addition, patients with elevated R-NLR were more likely to have a bigger tumor size and be female, while no association was found between elevated R-NLR and age, tumor location, differentiation, depth of infiltration, nodal involvement, TNM stage, vascular invasion, and neurological invasion. However, further research is required to verify these findings.
We also inducted a Cox model to identify the prognostic value of the preoperative R-NLR score, and after adjusting for other confounding factors, found that it remained an independent prognostic indicator for OS and DFS in stage II–III GC patients after radical surgery. Simultaneously, vascular invasion, depth of invasion, and TNM stage were also prognostic parameters.
The R-NLR score is a prognostic scoring system combining anemia status and the immune system, and our results demonstrated that the preoperative R-NLR score could as a powerful prognosticator compared to RDW or NLR alone for stage II–III GC patients following radical surgery and could be used to stratify patients at high risk of tumor recurrence to improve the survival estimation.
There are some limitations to this study. Firstly, its retrospective and single-center design, combined with the small number of patients may have led to bias in the results. Secondly, the collected prognostic indicators are limited, and thirdly, some patients received targeted drug therapy or second surgery after conventional chemotherapy, which may have skewed the results. In the future, prospective studies with larger sample sizes and full clinical staging are needed to validate our findings.
In conclusion, we demonstrated that the R-NLR score could act as an independent prognostic indicator in GC patients who received radical surgery. As the measurement of the R-NLR score is quick, easy, and non-invasive, it may be a useful prognostic indicator to assess the prognosis of stage II–III GC patients.
Acknowledgments
Thanks to The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital and all authors for their help.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-271
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jgo-21-271
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-271). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This article was approved by the Ethics Committee of The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital (No. KY2019177) and informed consent was obtained from participating patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. Erratum in: CA Cancer J Clin 2020;70:313. [Crossref] [PubMed]
- Mu GC, Huang Y, Liu ZM, et al. Application value of nomogram and prognostic factors of gastric cancer patients who underwent D2 radical lymphadenectomy. BMC Gastroenterol 2019;19:188. [Crossref] [PubMed]
- Bonelli P, Borrelli A, Tuccillo FM, et al. Precision medicine in gastric cancer. World J Gastrointest Oncol 2019;11:804-29. [Crossref] [PubMed]
- Wang Z, Wang J, Wang P. The prognostic value of prognostic nutritional index in hepatocellular carcinoma patients: a meta-analysis of observational studies. PLoS One 2018;13:e0202987 [Crossref] [PubMed]
- Ren Y, Wang Z, Xie J, et al. Prognostic value of the post-operative red blood cell distribution width in rectal cancer patients with neoadjuvant chemoradiation followed surgery. Biosci Rep 2020;40:BSR20201822 [Crossref] [PubMed]
- Wang P, Wang Z, Liu Y, et al. Prognostic value of platelet-associated biomarkers in rectal cancer patients received neoadjuvant chemoradiation: a retrospective study. Cancer Radiother 2021;25:147-54. [Crossref] [PubMed]
- Zhou C, Zhong X, Song Y, et al. Prognostic biomarkers for gastric cancer: an umbrella review of the evidence. Front Oncol 2019;9:1321. [Crossref] [PubMed]
- Hirahara N, Tajima Y, Fujii Y, et al. Comprehensive analysis of red blood cell distribution width as a preoperative prognostic predictor in gastric cancer. Anticancer Res 2019;39:3121-30. [Crossref] [PubMed]
- Li Y, Xing C, Wei M, et al. Combining red blood cell distribution width (RDW-CV) and CEA predict poor prognosis for survival outcomes in colorectal cancer. J Cancer 2019;10:1162-70. [Crossref] [PubMed]
- Shota S, Saito H, Kono Y, et al. Prognostic significance of pre- and post-operative red-cell distribution width in patients with gastric cancer. J Gastrointest Surg 2020;24:1010-7. [Crossref] [PubMed]
- Marano L, D'Ignazio A, Cammillini F, et al. Comparison between 7th and 8th edition of AJCC TNM staging system for gastric cancer: old problems and new perspectives. Transl Gastroenterol Hepatol 2019;4:22.
- Miyamoto R, Inagawa S, Sano N, et al. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol 2018;44:607-12. [Crossref] [PubMed]
- Yazici P, Demir U, Bozkurt E, et al. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark 2017;18:19-25. [Crossref] [PubMed]
- Jericó C, Osorio J, García-Erce JA, et al. Patient Blood Management strategies for iron deficiency anemia management in gastric cancer. Eur J Gastroenterol Hepatol 2019;31:547-8. [Crossref] [PubMed]
- Zhang FX, Li ZL, Zhang ZD, et al. Prognostic value of red blood cell distribution width for severe acute pancreatitis. World J Gastroenterol 2019;25:4739-48. [Crossref] [PubMed]
- Ferrucci L, Guralnik JM, Woodman RC, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med 2005;118:1288. [Crossref] [PubMed]
- Ma S, Song W, Xu Y, et al. Neutralizing tumor-promoting inflammation with polypeptide-dexamethasone conjugate for microenvironment modulation and colorectal cancer therapy. Biomaterials 2020;232:119676 [Crossref] [PubMed]
- Choi Y, Kim JW, Nam KH, et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer 2017;20:602-11. [Crossref] [PubMed]
- Macciò A, Madeddu C, Gramignano G, et al. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica 2015;100:124-32. [Crossref] [PubMed]
- Cho SY, Oh Y, Jeong EM, et al. Amplification of transglutaminase 2 enhances tumor-promoting inflammation in gastric cancers. Exp Mol Med 2020;52:854-64. [Crossref] [PubMed]
- Sun X, Liu X, Liu J, et al. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I-II gastric cancer. Chin J Cancer 2016;35:57. [Crossref] [PubMed]
- Rajwa P, Życzkowski M, Paradysz A, et al. Evaluation of the prognostic value of LMR, PLR, NLR, and dNLR in urothelial bladder cancer patients treated with radical cystectomy. Eur Rev Med Pharmacol Sci 2018;22:3027-37. [PubMed]
- Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019;8:886-894. [Crossref] [PubMed]
- Mainardi LG, Fernandes RC, Pimentel GD. The neutrophil-to-lymphocyte ratio is inversely associated with adductor pollicis muscle thickness in older patients with gastrointestinal tract cancer. Nutrition 2020;79-80:110887 [Crossref] [PubMed]
- Yu L, Lv CY, Yuan AH, et al. Significance of the preoperative neutrophil-to-lymphocyte ratio in the prognosis of patients with gastric cancer. World J Gastroenterol 2015;21:6280-6. [Crossref] [PubMed]
- Gun SY, Lee SWL, Sieow JL, et al. Targeting immune cells for cancer therapy. Redox Biol 2019;25:101174 [Crossref] [PubMed]
(English Language Editor: B. Draper)


