
The effect of the TP53 and RB1 mutations on the survival of hepatocellular carcinoma patients with different racial backgrounds
Introduction
With 0.78 million tumor-related deaths each year worldwide, liver cancer is the fourth leading cancer-related death (1). Hepatocellular carcinoma (HCC) accounts for 75–85% cases of liver cancer (1,2). Moreover, the incidence had ethnic difference as which in Asia is higher than that in Europe and America, both in men and women (1). The main risk factors for HCC include chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), aflatoxin, obesity/diabetes, and heavy alcohol intake (3). The prognosis for patients with HCC is poor, and HCC patients have a median survival time of approximately 11 months (4). Racial disparities in the survival of patients with HCC exist, and survival is significantly worse in African-American patients and superior in Asian patients, compared with white HCC patients (5-7). Disparities in disease progression raise further difficulties in the monitoring and treatment of HCC.
TP53 is one of the most commonly mutated genes in human cancer (8,9). A study of 12 cancer types with 3,281 tumor samples reported that the average mutation frequency of TP53 was about 42% (10). The wild-type (WT) TP53 plays an important role in cell cycle regulation and apoptosis after deoxyribonucleic acid (DNA) damage (11). Cells with DNA damage could escape from apoptosis in the absence of functional TP53 and transform into cancer cells (12). Mutations of TP53 are generally associated with poor prognosis. Indeed, several studies have reported that TP53 mutations are associated with poor survival in HCC patients (13-16). Additionally, evidence also suggests that the TP53 mutation is correlated with tumor stage, tumor differentiation, and vascular invasion in HCC (17,18).
RB1 is another frequently mutated tumor suppressor gene in HCC. RB1 functions as a negative regulator of cell cycle progression through the control of the E2 factor (E2F) family of transcription factors (19,20). Many transcription target genes of RB1/E2F are involved in DNA replication, DNA damage response, and cell cycle progression (21,22). Functional RB1 is important for the expression of these genes, and the disorder of RB1 is associated with cancer genesis and an aggressive cancer phenotype (23,24). In HCC, patients with an RB1 mutation have a poor prognosis (25).
The disruption of specific genes has profound effects on the responses of cells to genotoxic damage. The interaction of gene mutations with environmental and etiologic factors is important for carcinogenesis, and varies among geographic regions, and racial and ethnic groups (16). This study sought to explore the effects of gene mutations among patients with different racial backgrounds. Furthermore, we explored the relationship between mutations and survival among racial backgrounds. The sequencing and clinical data of 336 HCC patients were obtained from The Cancer Genome Atlas (TCGA) database and subject to a survival analysis. Ribonucleic acid (RNA)-sequencing data were extracted and used to analyze the infiltration of immune cells.
We present the article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-312).
Methods
Clinical cohorts
To analyze the relationship between gene mutations and HCC patients’ survival, the sequencing and clinical data of 336 HCC patients were obtained from TCGA website (https://portal.gdc.cancer.gov/repository) (up to March 20th, 2020) (26). Data on the overall survival (OS) of all patients and the disease-free survival (DFS) of 294 HCC patients were available. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Estimation of immune cell infiltration
Three hundred and twenty-four HCC samples had RNA-sequencing data available. CIBERSORT was used to assess the proportions of 22 infiltrating immune cell types, which contained myeloid subsets, plasma cells, natural killer (NK) cells, B cells, and 7 T cell types (27). This method used a leukocyte gene signature matrix comprising 547 genes to distinguish among different immune cell types. The results showed the proportion of each immune cell type, and the sum of all immune cell types was equal to 1.
Statistics analysis
R software was used to perform the statistical analysis. Kaplan-Meier curves were used to estimate OS and DFS. The log-rank test was used for the statistical analysis, and hazard ratios (HRs) were calculated using the Cox proportional hazards model. Wilcoxon rank-sum tests were performed to analyze the correlation between gene mutations and the proportion of infiltrating immune cells. Fisher’s exact tests were performed to analyze differences in gene mutation frequency among patients with different racial backgrounds. For the analysis, a P value below 0.05 was considered significant.
Results
Cohort characteristics
We used the public data set from TCGA of 336 HCC patients. The characteristics of TCGA HCC population are set out in Table 1. Patients were divided into two groups (i.e., Asian or Caucasian) according to race. Patients’ had mean ages (± standard deviation) of 55.0±11.6 and 63.8±13.2 in the Asian and Caucasian groups, respectively. The most prevalent history HCC risk factor was HBV in the Asian group (60.0%), and alcohol consumption in the Caucasian group (37.6%).
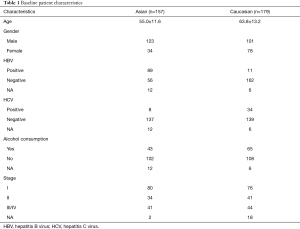
Full table
Gene mutation and survival
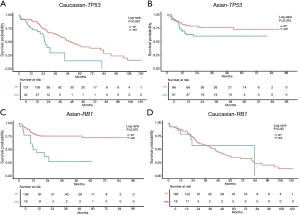
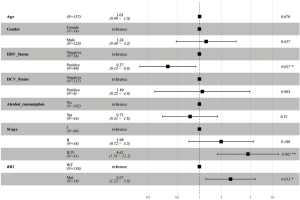
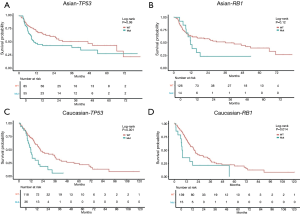
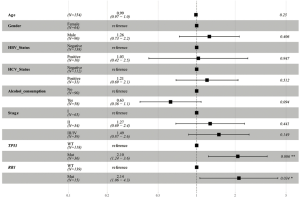
In this analysis, gene mutation contained missense, truncation, in-frame shift, fusion, and copy number variation. The most frequently mutated genes in the Asian group were TP53 (39%), CTNNB1 (26%), PCLO (12%), RB1 (12%), and ALB (11%) (see Figure 1). Conversely, the most frequently mutated genes in the Caucasian group were CTNNB1 (26%), TP53 (23%), ALB (15%), LRP1B (12%), and PREX2 (12%) (see Figure 1). TP53 was the only frequently mutated gene for which the mutation rate was significantly higher in Asian patients than Caucasian patients (39% vs. 23%; P=0.003). Caucasian patients with the TP53 mutation had significantly shorter OS [HR, 2.33; 95% confidence interval (CI), 1.36–3.97; P=0.002; see Figure 2A] than Asian patients with the TP53 mutation. However, there was no significant difference between WT TP53 and mutated TP53 in Asian patients (HR, 1.72; 95% CI, 0.95–3.14; P=0.075; see Figure 2B). In relation to the RB1 mutation, the results were reversed; that is, Asian patients with the RB1 mutation had an obviously shorter OS (HR, 3.37; 95% CI, 1.73–6.57; P<0.001; see Figure 2C) than Caucasian patients with the RB1 mutation. Indeed, the RB1 mutation had no effect on Caucasian patients’ OS (HR, 1.04; 95% CI, 0.45–2.4; P=0.924; see Figure 2D). To assess the independence of TP53 and RB1, we used a multivariable Cox proportional hazards model, adjusting for age, gender, HBV, HCV, alcohol consumption, and stage. After adjustments, the RB1 mutation was still found to be correlated with shorter OS (HR, 2.67; 95% CI, 1.23–5.8; P=0.013; see Figure 3) in Asian patients, and the TP53 mutation was found to be correlated with a shorter OS (HR, 2.11; 95% CI, 1.13–3.97; P=0.02; see Figure 4) in Caucasian patients. Thus, the RB1 and TP53 mutations appear to be independently associated with shorter OS in Asian and Caucasian patients, respectively.
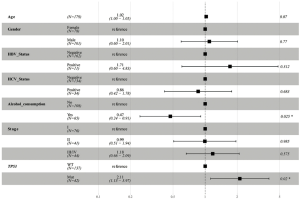
DFS data was available for a majority of the patients (140/157 Asian patients and 154/179 Caucasian patients). Thus, we also analyzed the relationship between gene mutations and patients’ DFS. The results of the statistical analysis for DFS differed to those for OS. Neither the TP53 (HR, 1.56; 95% CI, 0.98–2.5; P=0.061; see Figure 5A) nor the RB1 (HR, 1.71; 95% CI, 0.87–3.35; P=0.121; see Figure 5B) mutations correlated with significantly different DFS compared with WT in Asian patients. In Caucasian patients, both the TP53 (HR, 2.2; 95% CI, 1.38–3.51; P<0.001; see Figure 5C) and the RB1 (HR, 2.11; 95% CI, 1.15–3.88; P=0.017; see Figure 5D) mutations were associated with shorter DFS. We also used a multivariable Cox proportional hazards model to assess the independence of the TP53 and the RB1 mutations. After adjustment, the TP53 (HR, 2.10; 95% CI, 1.24–3.6; P=0.006; Figure 6) and RB1 (HR, 2.14; 95% CI, 1.06–4.3; P=0.034; Figure 6) mutations were still correlated with shorter DFS in Caucasian patients.
TP53 and RB1 mutations have different effects on Asian and Caucasian patients’ tumor microenvironments
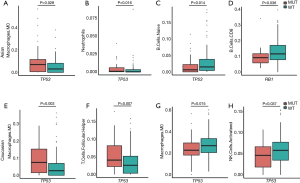
Using the CIBERSORT algorithm, we calculated the proportion of 22 infiltrating immune cell types in Asian and Caucasian HCC patients. Immune cell infiltration is key biomarkers to the prediction of clinical outcome and development of immunotherapies. Variations in the proportions of tumor infiltrating immune cells might reflect an intrinsic feature of different patients, and correlate with patients’ responses to treatments. Asian patients with mutated TP53 had relatively higher proportions of M0 macrophages and neutrophils (see Figure 7A,B), while the proportion of naïve B cells was decreased (see Figure 7C). The RB1 mutation in Asian patients was correlated with fewer infiltrating CD8 T cells in Asian patients (see Figure 7D). In relation to Caucasian patients, TP53 was correlated with increased proportions of M0 macrophages and follicular helper T cells (see Figure 7E,F). Additionally, the proportions of M2 macrophages and activated NK cells were decreased (see Figure 7G,H). However, no difference in immune cell infiltration was found between the RB1 mutation and WT sub-cohort in the Caucasian HCC patients. Thus, the TP53 and RB1 mutations appear to have divergent effects on immune infiltration in patients of different races.
Discussion
There was no major divergence in the frequently mutated genes of Asian and Caucasian HCC patients. Of the 17 most commonly mutated genes, only the mutation rate of TP53 was significantly higher in Asian HCC patients than Caucasian HCC patients (39% vs. 23%; P=0.003). Thus, carcinogenic-driver gene mutations in HCC patients are hardly affected by racial background.
Consistent with previous reports (15,28), Caucasian HCC patients with the TP53 mutation were found have significantly shorter DFS (HR, 2.2; 95% CI, 1.38–3.51; P<0.001) and OS (HR, 2.33; 95% CI, 1.36–3.97; P=0.002) than those with WT TP53. Several studies have indicated that Japanese (16,29,30) and Chinese (31) HCC patients with the TP53 mutation have a poor prognosis. However, in this study, we found that Asian patients with the TP53 mutation have a relatively shorter DFS (HR, 1.56; 95% CI, 0.98–2.5; P=0.061) and OS (HR, 1.72; 95% CI, 0.95–3.14; P=0.075) than those with WT TP53. However, these results, which are inconsistent with those of previous studies, may be attributable to the heterogeneity of Asian patients’ composition in America and the limited sample size (n=175).
To explore the intrinsic difference between these two groups of patients with the TP53 mutation, we used a CIBERSORT algorithm to calculate the proportions of 22 types of tumor infiltrating immune cells. Asian patients with the TP53 mutation had significantly higher proportions of M0 macrophages and neutrophils, and a lower proportion of naïve B cells. Tumor-associated neutrophils could recruit macrophages to promote the progression of HCC and are resistant to sorafenib (32). High levels of macrophages (33) and neutrophils (34) are associated with poor prognosis in HCC patients. In addition, a high density of tumor infiltrating naïve B cells was found to be associated with superior survival (35). These findings suggest that the TP53 mutation is associated with a poor prognosis in Asian patients. However, the survival analysis did not support this finding, as the results did not reach statistical significance. The use of proportions of immune cells and the small sample size of the present study limit interpretations of these results. An immunohistochemistry verification of the composition of the immune cells and a lager cohort for the survival analysis is needed. Caucasian patients with the TP53 mutation have increased proportions of M0 macrophages and follicular helper T cells, and decreased proportions of M2 macrophages and activating NK cells. The impaired function of activating NK cells (36,37) has been found to be associated with HCC progression and worse survival. Collectively, these results suggest that TP53 might play a role in the regulation of immune cell infiltration and is associated with poor prognosis in Caucasian HCC patients.
Preclinical research has reported that RB1 loss abrogates cell cycle control and genome integrity, and promotes liver carcinogenesis (23). In addition, clinical research has indicated that RB1 dysfunction is associated with worse survival in resectable HCC (25). Asian patients with the RB1 mutation have significantly shorter OS (HR, 3.37; 95% CI, 1.73–6.57; P<0.001) than those with WT RB1, but no such effect was observed among Caucasian patients (HR, 1.04; 95% CI, 0.45–2.4; P=0.924). The results in relation to DFS were reversed; that is, Caucasian patients with the RB1 mutation had significantly shorter DFS (HR, 2.11; 95% CI, 1.15–3.88; P=0.017), but no such effect was observed among Asian patients (HR, 1.71; 95% CI, 0.87–3.35; P=0.121). These results suggest that the RB1 mutation reduced the quality of lives of Caucasian patients and the survival time of Asian patients. Asian patients with the RB1 mutation had a significantly decreased proportion of CD8 T cells (P=0.036) than those WT RB1. The augmentation of CD8 T cells has been found to be associated with a favorable prognosis in HCC (38-40). A decreased proportion of CD8 T cells is consistent with a poor prognosis among Asian patients with the RB1 mutation.
It should be noted that this study had a number of limitations. First, the sample sizes of the Asian (n=157) and Caucasian (n=179) subgroups were limited. Second, although the clinical diagnosis and treatment methods of HCC in the East and the West are basically the same according to guideline, patients with different racial backgrounds may have different medical decisions due to economic reasons and different access to medical care, which may affect survival. Finally, proportions of infiltrating immune cells were used for comparisons; however, the sums of infiltrating immune cells varied among different patients; thus, quantitative analyses need to be undertaken.
To conclude, we observed that the TP53 and RB1 mutations had discordant effects on the survival of HCC patients with different racial backgrounds, exploring the correlations among gene mutations, races, immune microenvironment and clinical prognosis in HCC patients. In the research and treatment of HCC, more attention needs to be paid to geographic regions, racial, and ethnic groups.
Acknowledgments
Funding: Natural Science Foundation of Hunan Province (2018JJ3294); Natural Science Foundation of Hunan Province (2019JJ80007); Doctor Foundation Project of Hunan Provincial People’s Hospital (2020).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-312
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-312). TH, XS and JZ report that they are employees of OrigiMed. The other authors report funding from Natural Science Foundation of Hunan Province (Grant Number: 2018JJ3294), Natural Science Foundation of Hunan Province (Grant Number: 2019JJ80007) and Doctor Foundation Project of Hunan Provincial People’s Hospital (2020) during the conduct of the study.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. Erratum in: CA Cancer J Clin 2020;70:313. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011;15:223-43. vii-x. [Crossref] [PubMed]
- Greten TF, Papendorf F, Bleck JS, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 2005;92:1862-8. [Crossref] [PubMed]
- Davila JA, El-Serag HB. Racial differences in survival of hepatocellular carcinoma in the United States: a population-based study. Clin Gastroenterol Hepatol 2006;4:104-10; quiz 4-5. [Crossref] [PubMed]
- Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc 2006;98:1934-9. [PubMed]
- Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer 2010;116:1367-77. [Crossref] [PubMed]
- Hollstein M, Sidransky D, Vogelstein B, et al. p53 mutations in human cancers. Science 1991;253:49-53. [Crossref] [PubMed]
- Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science 2011;331:1553-8. [Crossref] [PubMed]
- Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333-9. [Crossref] [PubMed]
- Kastenhuber ER, Lowe SW. Putting p53 in context. Cell 2017;170:1062-78. [Crossref] [PubMed]
- Lai PB, Chi TY, Chen GG. Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepatocellular carcinoma cell line in vitro. Apoptosis 2007;12:387-93. [Crossref] [PubMed]
- Woo HG, Wang XW, Budhu A, et al. Association of TP53 mutations with stem cell-like gene expression and survival of patients with hepatocellular carcinoma. Gastroenterology 2011;140:1063-70. [Crossref] [PubMed]
- Ye S, Zhao XY, Hu XG, et al. TP53 and RET may serve as biomarkers of prognostic evaluation and targeted therapy in hepatocellular carcinoma. Oncol Rep 2017;37:2215-26. [Crossref] [PubMed]
- Honda K, Sbisà E, Tullo A, et al. p53 mutation is a poor prognostic indicator for survival in patients with hepatocellular carcinoma undergoing surgical tumour ablation. Br J Cancer 1998;77:776-82. [Crossref] [PubMed]
- Yano M, Hamatani K, Eguchi H, et al. Prognosis in patients with hepatocellular carcinoma correlates to mutations of p53 and/or hMSH2 genes. Eur J Cancer 2007;43:1092-100. [Crossref] [PubMed]
- Park NH, Chung YH, Youn KH, et al. Close correlation of p53 mutation to microvascular invasion in hepatocellular carcinoma. J Clin Gastroenterol 2001;33:397-401. [Crossref] [PubMed]
- Terris B, Laurent-Puig P, Belghitti J, et al. Prognostic influence of clinicopathologic features, DNA-ploidy, CD44H and p53 expression in a large series of resected hepatocellular carcinoma in France. Int J Cancer 1997;74:614-9. [Crossref] [PubMed]
- Nevins JR. The Rb/E2F pathway and cancer. Hum Mol Genet 2001;10:699-703. [Crossref] [PubMed]
- Sherr CJ. Principles of tumor suppression. Cell 2004;116:235-46. [Crossref] [PubMed]
- Zhu L. Tumour suppressor retinoblastoma protein Rb: a transcriptional regulator. Eur J Cancer 2005;41:2415-27. [Crossref] [PubMed]
- Vernell R, Helin K, Müller H. Identification of target genes of the p16INK4A-pRB-E2F pathway. J Biol Chem 2003;278:46124-37. [Crossref] [PubMed]
- Mayhew CN, Carter SL, Fox SR, et al. RB loss abrogates cell cycle control and genome integrity to promote liver tumorigenesis. Gastroenterology 2007;133:976-84. [Crossref] [PubMed]
- Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer 2019;19:326-38. [Crossref] [PubMed]
- Ahn SM, Jang SJ, Shim JH, et al. Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 2014;60:1972-82. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 2017;169:1327-41.e23. [Crossref] [PubMed]
- Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453-7. [Crossref] [PubMed]
- Liu J, Ma Q, Zhang M, et al. Alterations of TP53 are associated with a poor outcome for patients with hepatocellular carcinoma: evidence from a systematic review and meta-analysis. Eur J Cancer 2012;48:2328-38. [Crossref] [PubMed]
- Hayashi H, Sugio K, Matsumata T, et al. The clinical significance of p53 gene mutation in hepatocellular carcinomas from Japan. Hepatology 1995;22:1702-7. [Crossref] [PubMed]
- Sugo H, Takamori S, Kojima K, et al. The significance of p53 mutations as an indicator of the biological behavior of recurrent hepatocellular carcinomas. Surg Today 1999;29:849-55. [Crossref] [PubMed]
- Yuan RH, Jeng YM, Chen HL, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol 2006;209:549-58. [Crossref] [PubMed]
- Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology 2016;150:1646-58.e17. [Crossref] [PubMed]
- Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006;10:99-111. [Crossref] [PubMed]
- Wang Y, Yao R, Zhang L, et al. IDO and intra-tumoral neutrophils were independent prognostic factors for overall survival for hepatocellular carcinoma. J Clin Lab Anal 2019;33:e22872 [Crossref] [PubMed]
- Zhang Z, Ma L, Goswami S, et al. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. Oncoimmunology 2019;8:e1571388 [Crossref] [PubMed]
- Cariani E, Pilli M, Barili V, et al. Natural killer cells phenotypic characterization as an outcome predictor of HCV-linked HCC after curative treatments. Oncoimmunology 2016;5:e1154249 [Crossref] [PubMed]
- Kamiya T, Chang YH, Campana D. Expanded and activated natural killer cells for immunotherapy of hepatocellular carcinoma. Cancer Immunol Res 2016;4:574-81. [Crossref] [PubMed]
- Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007;132:2328-39. [Crossref] [PubMed]
- Kalathil SG, Hutson A, Barbi J, et al. Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight 2019;4:e130116 [Crossref] [PubMed]
- Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res 2016;4:419-30. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



