Adenocarcinoma associated with tail gut cyst
Introduction
Primary adenocarcinomas of the presacral (retrorectal) space are uncommon and usually arise from cystic lesions developing from remnants of the embryological postanal gut [tail gut cysts (TGC)] containing mucous-secreting epithelium. Clinical diagnosis is usually delayed by non-specific symptoms and histologic diagnosis is obtained after a biopsy or surgery. The potential for infection, perianal fistulas and perhaps most importantly, malignant change emphasizes that an early complete surgical resection is the therapy of choice.
Case report
A 56-year-old Caucasian female underwent an evaluation for an ovarian mass in 2005. She had a CT scan of abdomen/pelvis, which incidentally showed a presacral mass, which appeared cystic and measured 3 cm × 2.9 cm, in addition to the suspicious ovarian mass that required surgical removal. Although her operation was initially delayed for 6 weeks because of an episode of diverticulitis with pericolic abscess, she underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy and partial colonic resection. The presacral tailgut cyst (TGC) was left in place for unclear reasons.
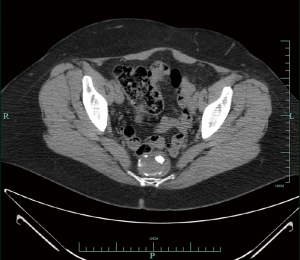
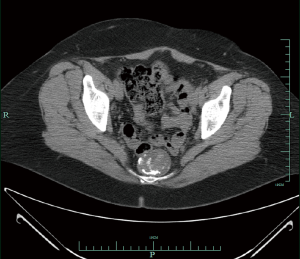
In 2008, she presented with hematuria, and a CT scan abdomen/pelvis revealed the cyst was larger, measuring 4.6 cm × 3.7 cm (Figures 1,2). A digital rectal examination gave the appreciation of a smooth mass. Further work-up included an endorectal ultrasound that revealed a smoothly marginated pre-sacral mass. Fine needle aspiration of the TGC in early 2009 was inconclusive, revealing only mucin and calcification. She then had surgery to remove the mass in toto in fall of 2009, by trans-sacral excision, using the technique of the Kraske procedure (posterior approach). On gross examination of the resected tissue, the TGC consisted of a disrupted sac-like structure, and measured 4.5 cm × 4 cm × 2.2 cm. The external surface was composed of soft, red-tan tissue. The histopathologic examination revealed presence of intestinal-type epithelium with dysplasia and invasive adenocarcinoma. Carcinoma was present in the muscle wall of the cyst without vascular or perineural invasion, and the margins of resection were uninvolved by carcinoma. Carcinoma was moderately differentiated, although there were some solid clusters (“tumor budding”), a feature regarded to have adverse prognostic significance in colorectal primaries.
Her medical history was significant for presence of Factor V Leiden with history of two episodes of deep vein thrombosis in lower extremities. Her family history was unrevealing for malignancies. She reported a 40-pack-year smoking history. She denied any constitutional symptoms, gastrointestinal and genitourinary symptoms. Physical examination was not significant for any abnormality.
Invasive carcinoma was found within the muscular wall of the cyst, and based on the origin in this ectopic site, it was not possible to provide a TNM stage. A whole body PET-CT scan done four months after surgery did not demonstrate any abnormal hypermetabolic activity to suggest metastatic disease. MRI pelvis also was unrevealing for any evidence of recurrent disease in the pelvis. Despite unclear utility in post-resection setting, serum CEA level was checked and was found to be within normal range. Over 1.5 years since the surgery, the patient has been carefully followed with periodic PET-CT scans, and has not received any further intervention-chemotherapy or radiation.
Discussion
TGC are rare congenital cysts that occur in the retrorectal space and are thought to arise from postanal primitive gut remnants (1). The retrorectal or presacral space is bounded anteriorly by the rectum, posteriorly by the sacrum, superiorly by the peritoneal reflection, inferiorly the levators ani and coccygeus muscles, and laterally by the ureters and iliac vessels (2). TGC have also been referred to as retrorectal cyst hamartoma (3), cyst of postanal intestine, tail gut vestiges, and rectal cyst (4). TGC should be distinguished from other lesions which may occur in the retrorectal space including teratomas, epidermal cysts, rectal duplication cysts, anal gland cysts, and anal gland carcinomas (4).
Although TGC may clinically present in all age groups from neonates to adults, the anomaly is more commonly found in middle-aged females. Most patients with TGC probably remain asymptomatic, and the cyst is discovered incidentally. When symptomatic, the presentation is usually non-specific and is most frequently related to compressive effects of a growing pelvic mass (e.g., rectal fullness, urinary frequency, rectal bleeding, pain on defecation, constipation, lower abdominal and back pain and symptoms associated with genitourinary obstruction). Infection, chronic abscesses and fistulas with the rectum or with perianal skin can also develop. The patient may present with a history of multiple drainage procedures for recurrent pilonidal abscess, perianal abscess or fistula-in-ano. Majority of TGCs are benign. However, malignant transformation of the epithelial component of a TGC has been reported on rare occasions. Malignancies that have been reported within TGC include adenocarcinomas, carcinoid tumors, neuroendocrine carcinomas, endometrioid carcinoma, adenosquamous carcinoma, squamous cell carcinoma and sarcoma (5,6). However, the majority is adenocarcinomas and carcinoid tumors. An extensive search of the literature revealed only 17 cases of adenocarcinoma arising in a TGC (1,3,6,7).
All TGC should be assessed for malignancy (5). Despite advances in a variety of diagnostic methods such as CT and MRI, a precise diagnosis can only be made by histopathologic examination after surgical removal (8). Although malignancy arising in a TGC has been reported with a needle biopsy, it is generally not advised as there is a potential for false-negative results and also, the biopsy carries the risk of spillage into the pelvic cavity and seeding of the biopsy tract. If the index of suspicion for malignancy is low and the patient is asymptomatic, routine surveillance may be appropriate. A transrectal or presacral needle biopsy may only be considered for patients who are at high surgical risk. Surgical extirpation, on the other hand, will provide an adequate specimen for definite histopathologic diagnosis and will serve as definitive treatment, in case carcinoma is present in TGC. In addition, surgery will also relieve the local symptoms and likely prevent infections and fistulae.
Adenocarcinoma arising in a TGC has occasionally been shown to be positive for CEA by immunohistochemistry, and has been associated with an elevated serum CEA and/or serum CA19-9. However, CEA elevation per se is not specific enough to permit a diagnosis of TGC adenocarcinoma. Nonetheless, once a TGC malignancy has been diagnosed and is associated with an elevated CEA, following CEA levels may be used as a simple measure to assess the tumor’s response to treatment or development of recurrence.
Imaging modalities such as CT provide additional clues in the differential diagnosis (9). CT scan shows a well-defined homogeneous retrorectal mass with the CT values ranging from water to soft-tissue density (10). Most TGC can be identified as multiloculated cysts on higher resolution scans. The keratinous or inflammatory debris within a cyst may account for a more solid appearance. Intralesional calcifications or bony destruction of coccyx or sacrum, commonly seen in sacrococcygeal teratomas, are usually absent in TGC. Because calcification is not a feature of TGC, its presence favors the diagnosis of a teratoma or malignancy. MRI imaging reveals a hypointense lesion on T1-weighted images and homogenously hyperintense lesions on T2-weighted images. Although the malignant portions of the tumor are characterized by irregular wall thickening and intermediate signal intensity on both T1- and T2-weighted images, MRI is probably not the best imaging modality to fully differentiate malignant from benign lesions.
The outcome of TGC-associated malignancies has varied from case to case. The factors that determine the prognosis have been thought to be the time of the diagnosis, completeness of resection and tumor histology and grade- neuroendocrine (or carcinoid) tumors having much better prognosis than adenocarcinomas. Many cases have reported the poor prognosis of adenocarcinoma arising from TGC due to local recurrence and metastases. Therefore, early surgical excision is recommended not only because it is difficult to determine if the TGC is benign or malignant, but also to allow definitive treatment of malignancy (8).
There is no general consensus on treatment standard nor are there any treatment guidelines for TGC-associated adenocarcinoma because of the very low incidence rate. However, anecdotal evidence of rapid, systemic failure has favored aggressive surgical treatment of TGC soon after their discovery (10). For the same reason, in some cases, adjuvant radiation therapy with or without chemotherapy has been employed with good outcomes (8). It can be hypothesized that adjuvant radiation and chemotherapy will sterilize the site of origin of carcinoma after removal, if there is a reasonable possibility that the tumor will recur. Another potential approach will be to clinically observe the patient with periodic PET-CT scans and serum CEA levels and monitor for signs of recurrence. However, it lacks evidence in the absence of a randomized controlled trial, with so few cases being reported. Despite that argument, we opted for the latter approach in our case after a thorough review of the available body of evidence and due discussion with the patient, of pros and cons of both the options. She has been followed clinically for a year and a half, with semi-annual PET-CT and MRI, and has not demonstrated any evidence of recurrent disease, locally or metastatic.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cho BC, Kim NK, Lim BJ, et al. A carcinoembryonic antigen-secreting adenocarcinoma arising in tailgut cyst: clinical implications of carcinoembryonic antigen. Yonsei Med J 2005;46:555-61.
- Jarboui S, Jarraya H, Mihoub MB, et al. Retrorectal cystic hamartoma associated with malignant disease. Can J Surg 2008;51:E115-6.
- Tampi C, Lotwala V, Lakdawala M, et al. Retrorectal cyst hamartoma (tailgut cyst) with malignant transformation. Gynecol Oncol 2007;105:266-8.
- Graadt van Roggen JF, Welvaart K, de Roos A, et al. Adenocarcinoma arising within a tailgut cyst: clinicopathological description and follow up of an unusual case. J Clin Pathol 1999;52:310-2.
- Au E, Anderson O, Morgan B, et al. Tailgut cysts: report of two cases. Int J Colorectal Dis 2009;24:345-50.
- Gönül II, Bağlan T, Pala I, et al. Tailgut cysts: diagnostic challenge for both pathologists and clinicians. Int J Colorectal Dis 2007;22:1283-5.
- Menteş BB, Kurukahvecioğlu O, Ege B, et al. Retrorectal tumors: a case series. Turk J Gastroenterol 2008;19:40-4.
- Maruyama A, Murabayashi K, Hayashi M, et al. Adenocarcinoma arising in a tailgut cyst: report of a case. Surg Today 1998;28:1319-22.
- Schwarz RE, Lyda M, Lew M, et al. A carcinoembryonic antigen-secreting adenocarcinoma arising within a retrorectal tailgut cyst: clinicopathological considerations. Am J Gastroenterol 2000;95:1344-7.
- Krivokapic Z, Dimitrijevic I, Barisic G, et al. Adenosquamous carcinoma arising within a retrorectal tailgut cyst: report of a case. World J Gastroenterol 2005;11:6225-7.