Impact of margin status and lymphadenectomy on clinical outcomes in resected pancreatic adenocarcinoma: implications for adjuvant radiotherapy
Introduction
Pancreatic ductal adenocarcinoma (PDA) carries one of the worst prognoses of all malignant neoplasms. Although its incidence is around 3% among all malignancies, it still resulted in 7% of all cancer deaths in 2014 within the United States (1). Over the last three decades, the survival rates of many cancers have had considerable improvement. However, the survival improvements in pancreatic cancer have been the worst among all malignances (1). The best clinical outcomes are noted in the 10-20% of patients with resectable tumors, yet even in this cohort, rates of recurrence remain high suggesting increased probability of occult regional and metastatic disease. Whereas metastatic failure predominate and are noted in up to 80% of patients, local recurrence still remains an important clinical consideration representing a component of failure in 50% of patients (2). As a result, various adjuvant strategies involving chemotherapy (CT) with or without radiotherapy (RT) have been investigated over the last few decades. Randomized clinical trials have validated the benefit of adjuvant CT with improved overall survival (OS) relative to no therapy (3,4). The addition of adjuvant RT, on the other hand, has been a point of debate considering the varying and often conflicting results and criticisms of EORTC 40891, ESPAC-1 and GITSG studies (5-8). These findings have prompted others to investigate pathologic variables that may predict patterns of failure and help further define the subset of patients who may benefit from adjuvant RT.
Previous studies have also recognized multiple independent variables that have prognostic significance including tumor staging, lymph node (LN) positivity, lymphovascular and perineural invasion, tumor grade and surgical resection margin involvement (9-12). Indeed, a recent meta-analysis investigating the benefit of adjuvant therapies in PDA suggested that adjuvant chemoradiation may have a clinically relevant benefit among patients with positive resection margin (4). The distance of margin clearance and its impact on local and regional failure rates also has not been clearly established, primarily due to the lack of a uniform definition for what constitutes margin positivity (13). LN involvement is also known to be prognostic and as a result, the extent of lymphadenectomy and LN ratio has been areas of active research and debate (9,14,15). Presently, there is no clear consensus or guidelines on the number of nodes that should be examined during PDA resection, as well as the prognostic significance of number and ratio of involved nodes. Some studies indicate that pathologic assessment of 12 or 15 nodes would provide the most accurate prognostic information, whereas others do not reveal a correlation between the numbers of nodes assessed and clinical outcomes (9,15,16). Additionally, there is very little data on whether the location of positive LN has any prognostic significance (17-19). Multiple randomized controlled trials have evaluated whether there is a survival benefit with extended resection, and most have not shown any significant survival benefit (20-23). Recent contemporary meta-analyses have also reaffirmed these findings (24,25). However, many of these trials and analyses have not specifically delineated whether periportal LN involvement from extended lymphadenectomy is prognostically relevant.
Herein, we have investigated the prognostic implications of comprehensive LN evaluation as well as surgical margin clearance in patients with resected PDA in order to assess the potential impact on clinical outcomes and their role in better defining the subset of resected patients who would benefit most from adjuvant chemoradiotherapy (CRT).
Definitions
It is has been difficult to determine the prognostic significance of margin clearance during PDA resection. This is further compounded by the lack of a consensus definition of what constitutes a positive margin. Since the late 1980s, the R classification has been adapted by the International Union Against Cancer (UICC) (26). Based on these guidelines, resection margins are classified as R0, no tumor at the resection margin, R1, microscopic tumor at the resection margin and R2, macroscopic tumor involvement of margins (27). In 2002, the Royal College of Pathologists, recommended that R1 be reclassified to include the presence of tumor cells within 1 mm of ink (28). To obscure the definition of R classification even further, clinical practice and institutional differences in pathological assessment protocols commonly differ in the application and utilization of the R1 classification till this day (13,29,30). Based upon these general guidelines for the purpose of this study, we categorized resection margins as positive (tumor at ink), ≤1 mm from ink, or >1 mm from ink.
On the other hand, pathologic evaluation of LNs has been more uniform. LN positivity is based on pathologic assessment of resected LNs, which show microscopic evidence of tumor. LN ratio (NR) is the number of positive nodes (NP) divided numbers of nodes examined (NE). Periportal LNs include those nodes that around the portal vein and hepatic artery.
Methods and materials
With institutional review board approval, we identified 1,545 patients from a prospective cancer database with the diagnosis of PDA and treated at a single tertiary care center since from 1990 to 2014. Of these, comprehensive clinical and pathologic data were retrospectively collected on 106 patients with stage I-III disease who underwent up front pancreaticoduodenectomy or distal pancreatectomy from 2007 to 2014.
Patient and disease specific data were obtained from electronic medical records and included consultation, clinic and procedure notes, endoscopic ultrasound, computed tomography, magnetic resonance imaging and positron emission tomography scans (Table 1). Post-operative disease-specific variables included type of adjuvant therapies, time to and location of recurrence, and date of death. Survival data was obtained from Medicare, SSDI, death certificates, physicians, hospice and family notification. Pathology reports of resected tumors were thoroughly analyzed for details of margin and LN involvement. Surgical resection margins were identified for pancreatic, uncinate, bile duct, vascular groove, anterior and posterior margins. The closest margin was recorded. Distance of tumor to ink was categorized as positive (tumor at ink), within 1 mm of ink, or greater than 1 mm from the inked margin. Additionally, LN characteristics included number of resected, positive and negative nodes, LN size and presence of periportal LN involvement.
Full table
In the first few years, since electronic pathology reports were available, involvement of periportal LN was not documented. Ultimately, twenty-five patients had documented periportal lymphadenectomy and histopathological evaluation of those LN.
All clinical and pathological data was collected and inputted into a new database for statistical analysis.
Statistics
One way analysis of variance (ANOVA) was used to compare the mean values of more than two continuous variables after appropriate data transformation when needed. Fisher’s exact test was carried out to test for association between two categorical factors. For time to event data such as OS and disease-free survival (DFS), the log-rank test was used to compare the survival distributions between different groups and the Cox proportional hazards model was used when the predictor was continuous. Multivariable Cox model was also used to adjust for confounders when assessing a predictor of interest. Standard variable selection techniques such as stepwise selection were used in the analysis. Model diagnostics and adequacy was assessed by testing the proportional hazards assumption graphically and analytically, and Martingale and Schoenfeld residuals were used to assess model adequacy.
Results
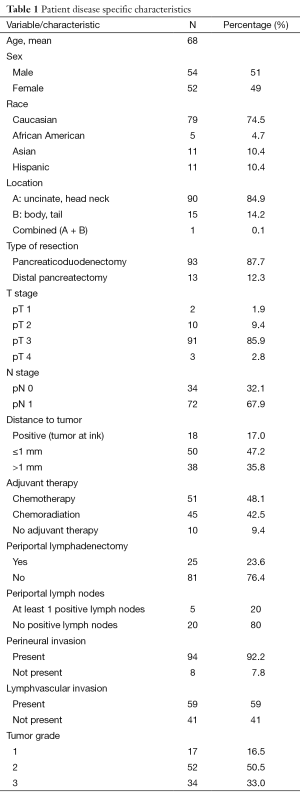
Median follow up was 11 months (range, 1-78 months). Of the 106 patients identified, 54 were men and median age for entire cohort was 69. Ninety-three patients underwent pancreaticoduodenectomy and 13 underwent distal resections. Resection margins were identified as positive (tumor at ink), ≤1 mm from ink, or >1 mm from ink in 18, 50, and 38 patients, respectively. A total of 48.1% of patients received adjuvant CT alone, while 42.5% received CRT and 9.4% received no adjuvant therapy. The median number of LNs resected was 19 (range, 4-37). Periportal LNs were resected in 25 of the 106 patients and were positive in 20%. Analysis of variance demonstrated that margin status was highly correlated with NP (P=0.012) and post-resection CA 19-9 (P=0.017) (Figure 1). The median disease-free survival (DFS) for the entire cohort was 336 days (11 months) and median OS was 482 days (16 months). Patients with resection margins that were positive, ≤1 mm, or >1 mm had a DFS of 9.64, 12.20 and 10.57 months (P=0.72), respectively and median OS of 13.15, 15.91 and 16.63 months, respectively (P=0.15).
On univariate analysis, NP (HR, 1.34; 95% CI, 1.01-1.79; P=0.0417), post-resection CA-19-9 above 90 (HR, 1.26; 95% CI, 1.01-1.58; P=0.039) and positive periportal LNs (HR, 5.00; 95% CI, 1.08-23.3; P=0.039) resulted in significantly lower median OS. Univariate analysis also showed similar lower median DFS with NP (HR, 1.68; 95% CI, 1.32-2.14; P<0.001), post-resection CA 19-9 above 90 (HR, 1.31; 95% CI, 1.09-1.58; P=0.0045) and positive periportal LNs (HR, 5.58; 95% CI, 1.45-21.5; P=0.012). Although univariate analysis did not reveal a significant difference in DFS regarding margin status, there was a trend towards increased DFS as the distance in margin increased from positive (tumor at ink), ≤1 to >1 mm.
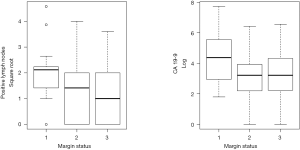
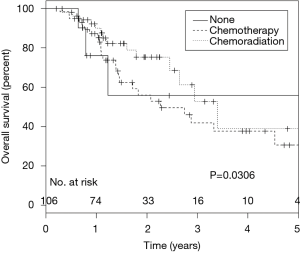
Margin status adjusted for NP was a significant predictor of DFS in patients receiving adjuvant CT alone with greater margin clearance leading to improved DFS (HR, 0.31; 95% CI, 0.09-0.99; P=0.048 for ≤1 mm, HR, 0.18; 95% CI, 0.05-0.66; P=0.01 for >1 mm, compared to positive), but did not result in a difference in OS (Figure 2). After controlling for other variables, including NP, the use of adjuvant CRT was associated with improved OS (P=0.03; HR =0.26; 95% CI, 0.08-0.88) in comparison to patients who received CT alone or no adjuvant therapy (Figure 3).
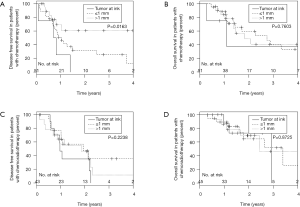
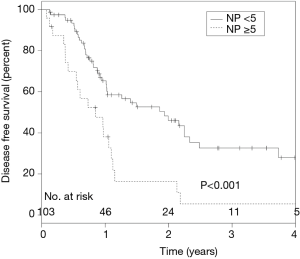
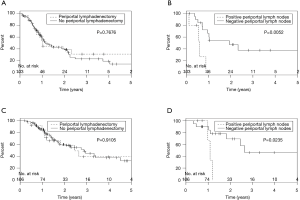
ROC analysis revealed that ≥5 NP to be the most accurate predictor of inferior DFS (P=0.005) (AUC =0.744). NE was not associated with DFS or OS, yet absolute NP of 5 or more was associated with a significantly worse DFS on multivariate analysis (HR, 2.5; 95% CI, 1.49-4.47; P≤0.001; Figure 4). A median NR of 0.11 was associated with inferior DFS (P=0.0043; HR =4.04), but was not associated with OS. Whereas periportal lymphadenectomy did not result in improved DFS or OS, patients with positive periportal LN had worse clinical outcomes (DFS, P=0.0052; OS, P=0.023) (Figure 5).
Discussion
Given the generally poor prognosis of resected pancreatic adenocarcinoma relative to other resected malignancies, histopathology variables, including microscopic resection margin distance, LN positivity and LN location may be able to provide a prognostic information to help guide the use of adjuvant therapy, particularly RT.
Similar to previous studies, we found that LN positivity and elevated post operative CA 19-9, individually portend a poor prognosis regarding disease free and OS. However, our study also provides insight into variables less frequently analyzed variables, such as involved periportal lymphadenopathy. Although multiple previous studies (20-25) have assessed clinical outcomes associated with extended lymphadenectomy, none specifically addressed whether the location of involved LNs removed during extended dissection offered prognostic value. Our study similarly concluded that extended dissection does not offer any survival benefit compared to standard dissection as periportal lymphadenectomy did not result in improved DFS or OS. However, contrary to previously reported data, we noted that periportal LN involvement was prognostic of a poor clinical outcome. In our cohort, periportal LN evaluation was limited to 25% of the patients. In spite of this limitation, the impact of positive periportal adenopathy on OS and DFS was quite significant. No patients with positive periportal LN were alive 1.5 years from diagnosis and DFS did not exceed 12 months. Whereas dissection of periportal LNs as part of pancreaticoduodenectomy remains an area of investigation, our data suggest that reporting of periportal LNs and associated involvement may provide crucial prognostic information. Many potential questions arise regarding the role of adjuvant therapy within this subgroup and we recommend periportal LN involvement be evaluated by larger and more comprehensive studies. Additionally, a standardized approach to evaluating these LNs should be considered.
Comprehensive LN analysis revealed ≥5 positive nodes to be the most accurate predictor of inferior DFS when analyzed as a continuous variable. Previous studies have shown a significant linear relationship between survival and up to 8 involved LNs, however the median nodes examined of that cohort was 17, where our median was 19 (9). In spite of this, absolute number of nodes examined was not associated with DFS or OS, mirroring similar results seen in Riediger et al. (15). Analysis of LN ratio showed a median of 0.11 was associated with inferior DFS, but did not impact OS.
Degree of margin clearance was an important predictor of DFS, particularly in patients receiving CT. Interestingly, this was not seen in the subgroup of patients receiving RT (Figure 2). A meta-analysis by Stocken et al. (4) revealed that CT resulted in improved clinical outcome only in the subgroup of patients with negative resection margins, and this benefit was mitigated in the presence of residual microscopic disease. Chang et al. (31) have hypothesized that the risk associated with local disease burden, in particular following an R1 resection, may not be overcome with CT alone, as this subset experienced clinical benefit following treatment with RT. Similar to our data, these results are further supported by the retrospective series by Herman et al. (32) which showed that irrespective of margin involvement, there was no difference in outcomes following receipt of adjuvant 5-FU based RT. Our results also suggest that reporting microscopic margin status at intervals of 1 mm distances may influence prognosis. The trend observed indicates that grouping of R0 and R1 resections according to the UICC definition maybe insufficient to properly stratify patients with PDA. Many previous studies have also had similar difficulty in showing the prognostic significance of R1 resection on both univariate and multivariate analysis (30). It is interesting to note that previous studies reporting resection margins vary vastly in the incidence of R1 resections (20% to 80%) (13). In our cohort, resection margins classified utilizing the Royal College of Pathologists and UICC definitions resulted in R1 resection rates of 64% and 17%, respectively, thereby underscoring the need for a universally accepted and clinically relevant definition of what constitutes a high-risk resection margin (13,30,33).
In our contemporary cohort, we also found that the addition of adjuvant RT to CT compared to those patients who received CT alone showed significant survival benefit. Multivariate analysis with margin status both included and removed in the statistical model provided similar outcomes with regards to OS. Previous meta-analysis (4) looking at the role of adjuvant RT in resected PDA had not shown significant benefit in OS, however they did suggest that among patients with positive resection margins, there was greater benefit of chemoradiation versus CT alone. We had relatively similar numbers of patients that received CT alone and chemoradiation and in our study population as whole, there was a clear clinical survival benefit by the addition of adjuvant RT. In a similar retrospective study by Sohn et al. (34) chemoradiation was found to a significant predictor of clinical outcome and mirroring our recent conclusions.
Despite our confirmatory findings and novel results, we recognize that there are limitations to our study. Our analysis was retrospective from a single institution experience. Some patients within our cohort lacked complete follow-up data, though this was small portion of the entire study population. In our evaluation of pathology reports, we found variability regarding the data reported. It was not until more recent pathological assessments that reporting had become more standardized. This is important because, a larger sample size with consistent margin reporting may reveal an even more significant impact on OS and DFS. Regardless, our study represents a contemporary evaluation of resected pancreatic adenocarcinoma patients and includes a broad analysis of multiple prognostic variables and their interrelationships.
Conclusions
In conclusion, we found that periportal nodal status is a valuable prognostic indicator that requires further investigation. With regards to margin status, we discovered that there is important and relevant clinical difference in evaluating margins at 1mm intervals and that the UICC definition of R1 resection maybe inadequate as a prognostic tool. Our data also suggests that the addition of adjuvant radiation therapy to CT in resected PDA, improves OS and this supports the role of RT in advanced PDA. Furthermore, the variability of resection rates and outcomes among previous studies, including our own, may in large part be due to the lack of a uniform histopathology evaluation protocol and margin resection definition. Our study supports the standardization of pathological evaluation of margin status and LN assessment among institutions. In the future, larger studies utilizing a standardized approach to histopathology evaluation may provide a clearer picture of which variables play a greater role in prognosis and help identify which subset of patients will benefit more from adjuvant CRT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg 2007;246:734-40. [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [PubMed]
- Stocken DD, Büchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer 2005;92:1372-81. [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [PubMed]
- House MG, Gönen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007;11:1549-55. [PubMed]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721-31; discussion 731-3. [PubMed]
- Meyer W, Jurowich C, Reichel M, et al. Pathomorphological and histological prognostic factors in curatively resected ductal adenocarcinoma of the pancreas. Surg Today 2000;30:582-7. [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer 2007;110:738-44.
- Verbeke CS. Resection margins in pancreatic cancer. Pathologe 2013;34 Suppl 2:241-7. [PubMed]
- Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol 2006;13:1189-200. [PubMed]
- Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg 2009;13:1337-44. [PubMed]
- Tomlinson JS, Jain S, Bentrem DJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg 2007;142:767-723; discussion 773-4.
- Krishna NB, Gardner L, Collins BT, et al. Periportal lymphadenopathy in patients without identifiable pancreatobiliary or hepatic malignancy. Clin Gastroenterol Hepatol 2006;4:1373-7. [PubMed]
- Meriggi F, Gramigna P, Forni E. Extended lymphadenectomy in cephalic pancreatoduodenectomy. Personal observations. Hepatogastroenterology 2007;54:549-55. [PubMed]
- Doi R, Kami K, Ito D, et al. Prognostic implication of para-aortic lymph node metastasis in resectable pancreatic cancer. World J Surg 2007;31:147-54. [PubMed]
- Jang JY, Kang MJ, Heo JS, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg 2014;259:656-64. [PubMed]
- Popiela T, Kedra B, Sierzega M. Does extended lymphadenectomy improve survival of pancreatic cancer patients? Acta Chir Belg 2002;102:78-82. [PubMed]
- Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 1998;228:508-17. [PubMed]
- Farnell MB, Pearson RK, Sarr MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 2005;138:618-28; discussion 628-30. [PubMed]
- Xu X, Zhang H, Zhou P, et al. Meta-analysis of the efficacy of pancreatoduodenectomy with extended lymphadenectomy in the treatment of pancreatic cancer. World J Surg Oncol 2013;11:311. [PubMed]
- Michalski CW, Kleeff J, Wente MN, et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg 2007;94:265-73. [PubMed]
- Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol Res Pract 1994;190:115-23. [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind Ch, editors. International Union Against Cancer TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell, 2009.
- Royal College of Pathologists. Standards and minimum datasets for reporting cancers. Minimum datasets for the histopathological reporting of pancreatic, ampulla of Vater and bile duct carcinoma. London: Royal College of Pathologists, 2002.
- Rau BM, Moritz K, Schuschan S, et al. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery 2012;152:S103-11. [PubMed]
- Campbell F, Smith RA, Whelan P, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009;55:277-83. [PubMed]
- Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855-62. [PubMed]
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [PubMed]
- Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008;15:1651-60. [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79. [PubMed]


