Stereotactic body radiotherapy for the pancreas: a critical review for the medical oncologist
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in men and women today and has a devastating prognosis with a dismal five year relative survival of seven percent (1). Due to poor diagnostic tools and the aggressiveness of PDAC, patients often present in the advanced stages of disease. If diagnosed in the early stages, the only curative and definitive treatment for PDAC is surgical resection or the Whipple procedure, which involves removal of the head of the pancreas, the duodenum, the gallbladder, a portion of the common bile duct, and in some cases, a part of the stomach. However, it is estimated that only 15–20% of patients initially present with surgically resectable PDAC (2). At the same time, despite controversies surrounding the role of radiation therapy in the treatment of PDAC due to conflicting results from clinical trials, chemoradiotherapy (CRT) is still consistently administered for PDAC, particularly in locally advanced cases (3,4).
Fractionated radiation therapy operates on the premise that normal tissue has the ability to repair its DNA after oxidative stress from radiation while malignant tissue, due to inherent genomic instability, cannot (5). Moreover, as opposed to delivering one massive radiation dose to the tumor, delivering the same radiation dose in smaller fractions is synergistically more potent in killing tumor cells and provides normal tissue the opportunity to regenerate and survive radiation. Traditionally, locally advanced PDAC has been treated with conventionally fractionated CRT, which involves delivering numerous small fractions of approximately 1.8–2 Gy daily over the course of many weeks. However, with advances in radiological imaging techniques and radiation treatment delivery, radiation oncologists have been able to deliver greater radiation doses accurately and precisely with brisk dose drop-off in neighboring structures through the advent of stereotactic body radiotherapy (SBRT) (6).
Throughout the last decade, SBRT has emerged as the mainstay of radiation treatment for certain cancers such as inoperable early stage non-small cell lung carcinoma and is currently under investigation for many other malignancies and cancer metastases including PDAC (7-12). In this review, we will discuss the general work flow in the setting of radiation oncology, the utilization and advantages of SBRT in the treatment of PDAC, and the future of radiation therapy in regards to tumor immunology in context of the general oncologist.
Overview of radiation therapy for PDAC patients treated with SBRT
Radiation oncology is a highly team-oriented specialty, and thus the process of receiving radiation therapy involves interactions between multiple members of the healthcare team. This involves the radiation oncologist, the dosimetrist, the physicist, the radiation therapist, and the nursing staff. Interactions with other specialties including palliative care, nutrition, medical oncology, surgical oncology, gastroenterology, and other subspecialties are important for both acute care and long term follow-up care as well. In patients who are in consideration for SBRT to the pancreas, often times at medical centers, the case is first reviewed at a multi-disciplinary tumor board. After the tumor board decides to proceed with SBRT, the patient is referred to radiation oncology for an initial consultation. Patients who will receive adjuvant SBRT after surgical resection of the lesion will need to wait four to six weeks before initiating radiation therapy in order to allow the tissue to heal.
At the consultation, the history of present illness is reviewed including all pertinent data. Critical family history specific to radiation oncology includes prior radiation therapy due to lifetime radiation dose exposure limits as well as a history of collagen vascular disease such as lupus or scleroderma because of the possible exacerbation of symptoms and serious long term toxicities after receiving radiation. Patients receiving SBRT to the pancreas also require an internal target for treatment accuracy. Typically patients will require fiducial marker placement in the pancreas endoscopically by a gastroenterologist (13,14). Referrals will be made accordingly. In patients with biliary stent placement, the placement of fiducials may be deferred depending on proximity to the lesion. With the placement of fiducials, patients typically have to wait at least one week before initiating treatment to allow the fiducials to heal and to limit fiducial movement during radiation (13,14). In addition, the radiation oncologist reviews medications, vitamins, and supplements taken as there is a theoretical detriment of radiation therapy with the use of megadoses of antioxidants (15). Patients also undergo laboratory tests to determine their BUN/creatinine and GFR because IV contrast is given during treatment planning. If the patient is premenopausal, a pregnancy test is routinely obtained with a discussion about contraception use during and after radiation.
After the initial consultation with the patient, the patient will schedule an appointment to undergo computer tomography simulation (CT-simulation). CT-simulation is a crucial step in radiotherapy because the scans obtained from the simulation are used for treatment planning. The difference between CT-simulation and a diagnostic CT scan is that the patient is placed in the desired treatment position using various body molds in order to guarantee reproducibility. Small tattoos are also placed on the patient in order to reproduce clinical set up using laser beams that are calibrated on both the CT scanner used during the simulation and the individual treatment linear accelerators. The goal of treatment planning is to identify the tumor volumes, the normal structures, and a radiation dose and coverage to the desired volume while avoiding dose tolerance limits to normal structures.
Since patients receiving SBRT to the pancreas receive large doses of radiation in a fewer number of fractions, special attention is needed for precision and accuracy. This is first achieved by immobilizing the patient by utilizing a custom-molded cushion, such as a vacuum fix, designed to increase patient comfort while minimizing movement and maximizing stability.
Despite limiting variability using custom-molds, mechanical respiration can also lead to changes in the position of the pancreas, often between one and three centimeters, causing variability in targeting (16,17). In order to account for these changes, patients are simulated with 4-dimentional computer tomography (4D-CT) simulation (18,19). 4D-CT simulators have the capability of simultaneously scanning the patient during the inspiratory phase of the respiratory cycle (20). This allows images to be obtained of the patient with maximal displacement. In addition, abdominal compression is used to mechanically limit movement. Intravenous contrast is often given during CT-simulation to help better characterize treatment volumes and parameters.
Common terminology for treatment planning include gross tumor volume (GTV), clinical tumor volume (CTV), internal target volume (ITV), and planning target volume (PTV). GTV is the visible target volume based on radiographic findings including those on CT, PET/CT, or other imaging modalities to delineate the tumor. In contrast, CTV includes areas beyond the target which could possibly contain microscopic disease in addition to the GTV. The CTV is often not uniform in shape because special attention is given to anatomical boundaries. For mobile targets such as the pancreas, the target volume is further enlarged to accommodate for target motility using the 4D-CT simulation, and this volume is defined as the ITV. In addition, due to mechanical errors and slight changes in patient positioning, the final target volume is expanded to account for these uncertainties, known as the PTV. In addition to the target tumor volumes, it is important to delineate non-target structures to limit the radiation dose to adjacent structures such as the stomach, small bowel, liver, kidneys, and spinal cord.
Once the physician finishes contouring, a treatment plan is created based on all of the gathered information, which includes the target volume, areas to avoid, and the radiation dose. In collaboration with the dosimetrist and the physicist, the radiation oncology team will formulate a treatment plan that will maximize the beam intensity and energy for the target while meeting the mandated conditions set forth by the radiation oncologist.
These parameters are then given to the dosimetrist who will then construct a treatment plan utilizing the calculated radiation dose, taking into consideration dose-limiting organs such as the small bowel and spinal cord. Dose constraints exist for each structure and are described as the TD50/5, whereby half of all patients receiving a certain dose to a specific tissue will develop fatal or severe complications within five years (21). Once the plan is created, it is then approved by the radiation oncologist, and the approved plan is reviewed by radiation oncology-certified physicists who verify the accuracy of the calculations for each treatment plan. In conjunction with approving treatment plans, these physicists are critical for maintaining the quality of the equipment and the safety of each patient and each treatment delivered. The treatment plan is then carried out by the radiation therapist. Typically, radiation planning takes one to two weeks.
SBRT treatment for the pancreas can vary between fractions from as little as one fraction to as many as five fractions (22). Patients are set up for treatment based on their setup images from CT-simulation. The radiation from SBRT can be delivered using a variety of linear accelerators. After immobilizing the patient, cone-beam CT scans or X-ray images, known as portal radiographs, are acquired before initiating treatment and are compared to the setup images obtained during the simulation to assess for positioning and for quality assurance purposes. The patient’s position is then fine-tuned until both collections of images fully correspond with each other with respect to soft tissue as well as the position of the fiducial or stent.
Throughout the course of treatment, nurses work alongside patients as their advocate, and patients are seen on a weekly basis while receiving radiation. Continuous follow up after completing radiation therapy has been an integral component of the radiation oncologist’s duties. Radiation side effects often range from fatigue, nausea, and vomiting to ulcerations and small bowel perforation in rare cases. Given that SBRT is usually given in a short course, sometimes these symptoms may present or persist after the completion of radiation treatment.
Initial follow up is important to manage the acute effects of treatment and to monitor normal recovery from therapy, while follow up in the long run is critical to assess for the late effects of treatment. Often patients are seen one month after treatment, and a follow up plan is created for the patient to see his/her medical oncologist and surgical oncologist for further evaluation.
The usage of SBRT in the pancreas
Even with advancements in novel treatments for PDAC, the overall survival for patients with PDAC is grim. Thus, new treatment modalities and technologies continue to be developed for PDAC such as SBRT, which has been studied in great depth in recent years.
The first group to report on the use and efficacy of SBRT in the treatment of PDAC was at Stanford University in 2004 in a phase I study (23). The study consisted of prescribing a single fraction of either 15, 20, or 25 Gy to patients with PDAC and found that a dose of 25 Gy was sufficient to achieve local control with no acute GI toxicities higher than grade 2 (23). Since then, many other studies have examined the outcomes and dosing regimens of SBRT and chemotherapy-SBRT-combined treatment modalities for PDAC (11,23-30). In these studies, local control rates ranged from 57% to 100%, with median overall survival reported between 5.7 and 20 months and total delivered doses ranging from 25 Gy in one fraction to 45 Gy in five fractions (Table 1). All of these studies examined the outcomes of single fraction and multi-fraction SBRT on PDAC, and a recent report demonstrated that while the efficacy of single fraction and multi-fraction SBRT was comparable, SBRT-related toxicities were decreased in patients receiving multi-fraction SBRT (31).
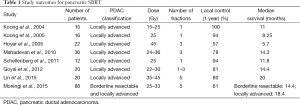
Full table
The limiting factor in the treatment of PDAC with radiation therapy is not necessarily the tumor itself but the dose-limiting adjacent structures to the target such as the duodenum and stomach and related toxicities. This is partially due to the relative radioresistance of the pancreas compared to neighboring structures and is particularly important for PDAC originating at the head of the pancreas as it often compresses the duodenum. Although SBRT is generally well tolerated during treatment, a number of studies have noted an increase in grade 3 toxicities in the months following the completion of radiation, namely gastroduodenal ulcerations, GI bleeding, small bowel perforations, and biliary strictures (24,26-29) (Table 2). Other studies have attributed these side effects to the dose and volume of radiation to surrounding organs (32-34). Because of the mobile nature of the pancreas, further reports have elucidated the role of respiratory motion and radiation dose received by the duodenum due to the compression of abdominal structures during inspiration (35). However, it is important to note that despite these toxicities, SBRT is still generally well tolerated, appears to improve the quality of life, and alleviates pain following treatment (28,36).
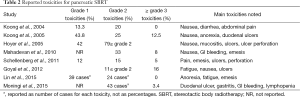
Full table
Related to issues with quality of life, because PDAC has a poor prognosis and patients often present in the advanced stages of disease, the main advantage of SBRT is the short course of radiation treatment, giving patients the opportunity to appreciate and savor their remaining time earlier. Lastly, SBRT has the potential to be utilized in palliative settings and inhibit gastric outlet obstruction (37).
Future directions
With constant advancements in chemotherapy and radiation therapy, SBRT provides solid local control rates for PDAC and has demonstrated encouraging results in the treatment of many different malignancies (11,23-30). Recently, much attention has been given to the tumor microenvironment, and the field of oncology has seen an explosion of therapeutic agents targeting the tumor microenvironment and tumor immunology.
The tumor microenvironment is a complicated entity that has been strongly implicated in the pathogenesis, proliferation, and invasive behaviors of several cancers (38-42). Tumors are often described in the literature as “dying” cells that have the capacity to evade the host immune response. Among many other reasons, this concept partially stems from the fact that solid tumors have been found to express negatively charged phospholipids, namely phosphatidylserine (PS), on the outer surface of their cell membranes and in tumor vasculature (43). Typically only expressed on the surface of dying cells, negatively charged phospholipids such as PS are exclusively present on the inner leaflet of the plasma membrane, and the expression of PS on the cell membrane mediates suppression of the host immune response against the tumor cells. Bavituximab is a chimeric antibody that specifically binds to PS expressed on the plasma membrane of these solid tumors, reactivating the host immune system against the tumor and has demonstrated promising results in clinical trials (44-46). Likewise, PS exposure is also increased with chemotherapy and radiation therapy, and future research should determine whether a synergistic effect exists between bavituximab, chemotherapy, and radiation therapy (43).
Other immunomodulatory classes of drugs currently under investigation are programmed cell death protein 1 (PD-1) inhibitors and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) inhibitors. PD-1 is expressed on the surface of T cells, causing immune suppression after binding to programmed death ligand 1 (PD-L1), which is occasionally expressed on the surface of tumor cells. CTLA4 is also expressed on the surface of T cells and downregulates the immune system by interacting with B7-1 and B7-2 on antigen presenting cells (APCs). Both PD-1 and CTLA4 inhibitors prevent binding of PD-1 and CTLA4 to their normal ligands, thereby limiting the immunosuppressive effects of these interactions. Nivolumab and pembrolizumab are two such PD-1 inhibitors, and ipilimumab and tremelimumab are two CTLA4 inhibitors currently being examined. The use of these drugs—singly, in combination together, or with traditional chemotherapy—has generated exceedingly favorable results with few highly potent side effects (47-51).
These encouraging data from tumor immunotherapies paves the way for the feasibility of combining these targeted molecules with radiation therapy. The abscopal effect describes the elimination and prevention of tumor metastases through radiation therapy, which has been reported in the literature (52-54). Stemming from the abscopal effect, the hope and expectation would be that tumor antigens released during radiation treatment could immobilize the host immune response through these tumor immunotherapies (55). Therefore, the opportunity to combine these immune modulatory agents with SBRT offers exciting prospects for the development of future PDAC treatment regimens.
SBRT has gained enormous attention within the last decade, and a growing body of assuring evidence presents tremendous opportunities for future investigations in this area. With an increasing number of studies over time, the field will have a greater understanding of radiation dose delivery through SBRT while maximally limiting adverse toxicities. This will lead to better and more complete information regarding outcomes and quality of life with SBRT and will aid to elucidate the role of SBRT and radiation in the treatment of PDAC. Furthermore, the possibility of examining novel combined immunotherapy-SBRT treatment regimens will allow oncologists from various medical fields to more intimately collaborate and interact moving forward.
Acknowledgements
Funding: David P. Horowitz is supported by RSNA Research and Education Foundation Grant RR1321.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004;363:1049-57. [Crossref] [PubMed]
- Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J Gastrointest Oncol 2011;2:157-67. [PubMed]
- Katz MH, Landry J, Kindler HL. Current controversies in the stage-specific multidisciplinary management of pancreatic cancer. Am Soc Clin Oncol Educ Book 2014.e157-64. [Crossref] [PubMed]
- Steel GG, Adams GE, Peckham MJ, editors. The Biological Basis of Radiotherapy. Amsterdam: Elsevier Science Publishers, 1983:181-95.
- Trakul N, Koong AC, Maxim PG, et al. Modern radiation therapy techniques for pancreatic cancer. Gastroenterol Clin North Am 2012;41:223-35. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Quero L, Hennequin C. Stereotactic radiotherapy for prostate cancer. Cancer Radiother 2014;18:332-6. [Crossref] [PubMed]
- Scorsetti M, Clerici E, Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol 2014;5:190-7. [PubMed]
- Amini A, McDermott JD, Gan G, et al. Stereotactic body radiotherapy as primary therapy for head and neck cancer in the elderly or patients with poor performance. Front Oncol 2014;4:274. [Crossref] [PubMed]
- Moningi S, Dholakia AS, Raman SP, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol 2015;22:2352-8. [Crossref] [PubMed]
- Bhattacharya IS, Woolf DK, Hughes RJ, et al. Stereotactic body radiotherapy (SBRT) in the management of extracranial oligometastatic (OM) disease. Br J Radiol 2015;88:20140712. [Crossref] [PubMed]
- Sanders MK, Moser AJ, Khalid A, et al. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc 2010;71:1178-84. [Crossref] [PubMed]
- Varadarajulu S, Trevino JM, Shen S, et al. The use of endoscopic ultrasound-guided gold markers in image-guided radiation therapy of pancreatic cancers: a case series. Endoscopy 2010;42:423-5. [Crossref] [PubMed]
- Lawenda BD, Kelly KM, Ladas EJ, et al. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst 2008;100:773-83. [Crossref] [PubMed]
- Mori S, Hara R, Yanagi T, et al. Four-dimensional measurement of intrafractional respiratory motion of pancreatic tumors using a 256 multi-slice CT scanner. Radiother Oncol 2009;92:231-7. [Crossref] [PubMed]
- Minn AY, Schellenberg D, Maxim P, et al. Pancreatic tumor motion on a single planning 4D-CT does not correlate with intrafraction tumor motion during treatment. Am J Clin Oncol 2009;32:364-8. [Crossref] [PubMed]
- D'Souza WD, Nazareth DP, Zhang B, et al. The use of gated and 4D CT imaging in planning for stereotactic body radiation therapy. Med Dosim 2007;32:92-101. [Crossref] [PubMed]
- Mancosu P, Bettinardi V, Passoni P, et al. Contrast enhanced 4D-CT imaging for target volume definition in pancreatic ductal adenocarcinoma. Radiother Oncol 2008;87:339-42. [Crossref] [PubMed]
- Keall PJ, Joshi S, Vedam SS, et al. Four-dimensional radiotherapy planning for DMLC-based respiratory motion tracking. Med Phys 2005;32:942-51. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Trakul N, Koong AC, Chang DT. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin Radiat Oncol 2014;24:140-7. [Crossref] [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [Crossref] [PubMed]
- Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005;76:48-53. [Crossref] [PubMed]
- Lin JC, Jen YM, Li MH, et al. Comparing outcomes of stereotactic body radiotherapy with intensity-modulated radiotherapy for patients with locally advanced unresectable pancreatic cancer. Eur J Gastroenterol Hepatol 2015;27:259-64. [Crossref] [PubMed]
- Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2010;78:735-42. [Crossref] [PubMed]
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72. [Crossref] [PubMed]
- Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:181-8. [Crossref] [PubMed]
- Goyal K, Einstein D, Ibarra RA, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res 2012;174:319-25. [Crossref] [PubMed]
- Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2005;63:320-3. [Crossref] [PubMed]
- Pollom EL, Alagappan M, von Eyben R, et al. Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: outcomes and toxicity. Int J Radiat Oncol Biol Phys 2014;90:918-25. [Crossref] [PubMed]
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86. [Crossref] [PubMed]
- Bae SH, Kim MS, Cho CK, et al. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys 2012;84:e469-74. [Crossref] [PubMed]
- Bae SH, Kim MS, Kim SY, et al. Severe intestinal toxicity after stereotactic ablative radiotherapy for abdominopelvic malignancies. Int J Colorectal Dis 2013;28:1707-13. [Crossref] [PubMed]
- Taniguchi CM, Murphy JD, Eclov N, et al. Dosimetric analysis of organs at risk during expiratory gating in stereotactic body radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 2013;85:1090-5. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Tkachev SI, Medvedev SV, Znatkova YR, et al. Possibilities of stereotactic radiotherapy in the palliative treatment of patients with pancreatic cancer. Vopr Onkol 2015;61:121-4. [PubMed]
- Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 2008;68:918-26. [Crossref] [PubMed]
- Tsujino T, Seshimo I, Yamamoto H, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res 2007;13:2082-90. [Crossref] [PubMed]
- Hayward SW, Wang Y, Cao M, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 2001;61:8135-42. [PubMed]
- Romaniuk A, Lyndin M. Immune microenvironment as a factor of breast cancer progression. Diagn Pathol 2015;10:79. [Crossref] [PubMed]
- Shi L, Wang L, Hou J, et al. Targeting roles of inflammatory microenvironment in lung cancer and metastasis. Cancer Metastasis Rev 2015;34:319-31. [Crossref] [PubMed]
- Thorpe PE. Targeting anionic phospholipids on tumor blood vessels and tumor cells. Thromb Res 2010;125 Suppl 2:S134-7. [Crossref] [PubMed]
- Gerber DE, Stopeck AT, Wong L, et al. Phase I safety and pharmacokinetic study of bavituximab, a chimeric phosphatidylserine-targeting monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 2011;17:6888-96. [Crossref] [PubMed]
- Digumarti R, Bapsy PP, Suresh AV, et al. Bavituximab plus paclitaxel and carboplatin for the treatment of advanced non-small-cell lung cancer. Lung Cancer 2014;86:231-6. [Crossref] [PubMed]
- Chalasani P, Marron M, Roe D, et al. A phase I clinical trial of bavituximab and paclitaxel in patients with HER2 negative metastatic breast cancer. Cancer Med 2015;4:1051-9. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol 2014;25:1750-5. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [Crossref] [PubMed]
- Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795-803. [Crossref] [PubMed]
- Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014;2:831-8. [Crossref] [PubMed]


