
The relationship between the number of circulating tumor cells and the prognosis in patients with esophageal squamous cell carcinoma
Introduction
Esophageal cancer (EC) is one of the most common malignancies worldwide, with high morbidity and mortality rates (1). Although the management and treatment of patients with EC have improved over the preceding decades, the 5-year overall survival rate remains inadequate. Various risk factors may be associated with poor prognosis of EC patients, including demographics (age, gender, or race/ethnicity), smoking, alcohol consumption, gastroesophageal reflux disease, diet, obesity and body composition, etc. This draws urgent attention to the need for effective detection and prediction approaches before the treatment of EC. The disease often goes undiagnosed until it reaches an advanced stage due to its atypical early clinical manifestations (2). Therefore, it is necessary to identify novel targets of early diagnosis, prognosis prediction, and therapeutic strategies for patients with EC.
Recently, circulating tumor cells (CTC) regarded as the main cause of tumor metastasis or recurrence. Cancer cells that have shed from the primary tumor are able to invade into surrounding tissues, to intravasate into the bloodstream to become CTCs. At least one part of CTCs will be able to generate distant metastases. CTCs are the type of tumor cells shed from a primary or metastatic tumor and are detected in the peripheral blood. CTCs are genetically and phenotypically heterogeneous with the primary tumor, so the detection of CTCs in transit through the body by a simple blood draw may not only provide useful information for early tumor diagnosis and possible treatment strategy but may also help to update the understanding of tumor biology and eventually improve the life quality of patients with different cancers (3). Previous studies have confirmed a close correlation between high levels of CTCs and the progression of several tumors in cancers such as breast cancer (4), colorectal cancer (5), gastric cancer (6), lung cancer (7), and EC (8). This suggests that high levels of CTCs are related to a worse prognosis. Research into CTCs in EC is relatively scarce, and most of the studies lack long-term follow-up.
The Kit gene, a proto-oncogene, is proven to be involved in gastrointestinal stromal tumors (9), mast cell disease (10), melanoma (11), and so on. The overexpression of Kit is associated with poor outcomes in tumor patients. However, the relationship between the Kit gene and EC remains elusive. In the present study, we analyzed the relationship between CTC levels, epithelial/mesenchymal cell types, Kit expression, and prognosis in patients with EC. We present the following article in accordance with the REMARK reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-409).
Methods
Tissue specimens
A total of 43 EC patients who underwent surgical resection (R0 resection) from June 2016 to January 2017 were recruited. The inclusion criteria were as follows: the patients underwent R0 resection, had no history of other malignant tumors, and no history of anti-tumor treatments such as radiotherapy, chemotherapy, immunotherapy, biotherapy, etc. Patients with a history of blood transfusion were excluded. Tissue specimens were checked by routine pathological examination and classified based on the EC staging criteria released by UICC/AJCC (8th edition). Peripheral blood CTC tests were performed twice before surgery and 1 week after surgery.
Regular follow-ups were made every 3 months after surgery. The follow-up endpoint of recurrence or metastasis came about if the tumor recurred or metastasized or 3 years after surgery. The endpoint of survival evaluation was death or 3 years after surgery. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethical Committee of the First Medical Center,General Hospital of Chinese PLA (No. 2021-147-03) and written informed consent was obtained from all patients.
CTCs separation
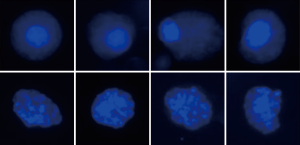
The CanPatrol@ assay (Yishan, Guangzhou) was applied to detect CTCs in peripheral blood in this study. Peripheral blood of clinical patients was collected as samples. Firstly, the red blood cells were lysed in a preservation solution, then passed through a nanofiltration membrane. Due to different cell sizes of white blood cells (smaller) and tumor cells (larger), tumor cells with larger diameters could not pass through the membrane and could not be collected. Subsequently, multiple RNA in situ hybridization (RNA-ISH) was used to identify and classify the collected CTCs. RNA-ISH results were observed by a semi-automatic fluorescence microscope; microscopic features of the CTC karyotype were represented in Figure 1. After the RNA-ISH and staining, different antibodies with specific-color fluorescence signals were used to classify the CTCs. The Classification criteria of CTCs were shown in Table 1.

Full table
Detection of c-Kit gene expression in CTCs
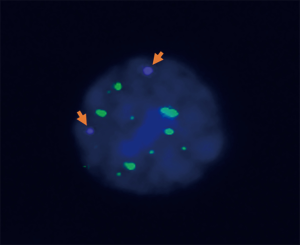
The expression levels of c-kit in CTCs were detected after collection. Multiple probes (probe working solution) were added to in situ hybridizations of multiple RNAs. CD45 probes labeled leukocytes for reverse screening, showing the white signal under fluorescence microscope if positive; epithelial CTCs were labeled with EpCAM and CK8/18/19 probes and displayed a red signal when positive; interstitial CTCs were labeled with a Vimentin probe and Twist probe to display a green signal; the positive c-Kit probe displayed a purple signal. DAPI was used to stain the nuclei blue (Figure 2).
Statistical analysis
Statistical evaluation was performed using SAS software (Version 9.4, SAS Institute, Inc., Cary, North Carolina, USA). The continuous variables and the number of CTCs were expressed as mean ± standard error or median (interquartile spacing), the classified variables were provided as a frequency (percentage). Associations and differences among the different parameters were analyzed using the t-test or Mann-Whitney U test, and the chi-square test or Fisher’s exact test was performed for classification variables.
Multivariate logistic regression analysis was performed, and the odds ratio (OR) was adjusted for gender, family history, and other factors. The Cox proportional hazards regression model was used to calculate mortality hazard ratios and 95% confidence intervals (CIs). The Kaplan-Meier method and log-rank test were used for survival analyses. A P value <0.05 was considered as statistically significant.
Results
Baseline characteristics
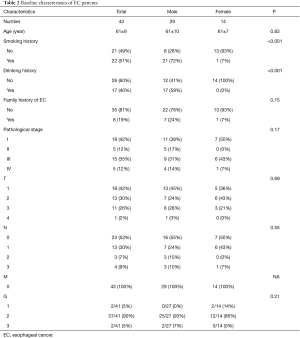
A total of 43 EC patients who had undergone R0 resection were enrolled in this study, made up of 29 males (67.4%) and 14 females (32.6%). The mean age of patients at diagnosis was 61 years old. Among them, there were 22 (51.2%) smokers and 21 (48.8%) non-smokers. Based on postoperative pathology and TNM staging, 18 (41.9%) patients were in stage I, 5 (11.6%) were in stage II, 15 (34.9%) were in stage III, and 5 (11.6%) were in stage IV (Table 2).
Full table
Detection rates of CTCs with epithelial, mixed, and stromal types in EC patients
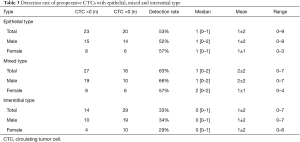
Among all 43 patients, 23 patients displayed epithelial-type CTCs (53%), with the detected range from 0 to 9; 27 patients displayed mixed CTCs (63%, range from 0
Full table
The relationship between different types of CTCs and pathological stages
The EC patients were divided into groups based on their number of CTCs. Among these, CTCs (epithelial + mixed + interstitial) were grouped into ≤2 and >2; CTCs of epithelial and mixed type were grouped into ≤1 and >1, and interstitial CTCs were grouped into 0 and >0.
For patients with CTCs (epithelial + mixed + interstitial), logistic regression showed that the OR (95% CI) of stage III/IV patients with more than 2 CTCs was 2.29 (0.67

Full table
Similarly, in patients with epithelial-type CTCs, the OR (95% CI) of stage III/IV patients displaying more than 1 detected epithelial CTC was 0.40 (0.09
In univariate analysis, the OR (95% CI) of stage III/IV patients with more than 1 mixed CTC was 1.87 (0.55
As for interstitial-type CTCs patients, the results of the univariate analysis showed the OR (95% CI) of stage III/IV patients with CTCs >0 was greater than the group where CTCs =0, which was 1.89 (0.52
Analysis of changes in pre- and post-operation CTCs
In the present study, we analyzed the CTCs in the peripheral blood of patients pre- and post-operation. The results were shown as follows: following operation, there were 15 patients displaying a positive result for epithelial CTCs (34.9%); 22 patients displaying mixed CTCs (51.2%); interstitial CTC results were positive in 11 patients (25.6%). The total number of CTC-positive patients was 30 (69.8%). Among them, the positive rate of mixed-type CTCs represented a significant difference before and after surgery. Other subtypes of CTCs showed a decrease without statistical significance (Table 5).
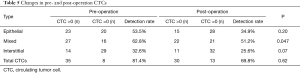
Full table
The relationship between CTC types and recurrence/metastasis in patients
The classification of EC patients with different types of CTCs was the same as previously described, namely, CTCs (epithelial + mixed + interstitial) were grouped into ≤2 and >2; CTCs of epithelial and mixed type were grouped into ≤1 and >1, and the interstitial CTCs were grouped into 0 and >0.
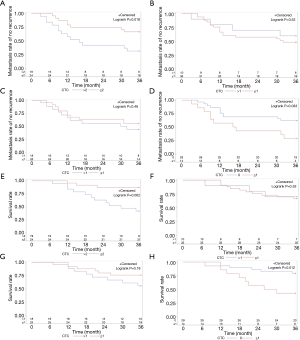
The results of Kaplan-Meier survival analysis and the log-rank test showed that the risk of post-operative recurrence and metastasis was significantly increased in patients within the high CTCs group (P=0.018, Figure 3A). As for patients with epithelial-type and mixed-type CTCs, no statistically significant difference was found in the risk of postoperative recurrence and metastasis between the two groups (P=0.53, 0.46, respectively; Figure 3B,3C). In patients with interstitial-type CTCs, the risk of postoperative recurrence and metastasis was increased in the CTCs >0 group (P=0.033, Figure 3D).
The relationship between CTC types and postoperative survival of patients
Among the total 43 patients, 14 patients died 3 years later, demonstrating a 3-year overall survival rate of 67%. The group classification remained the same as in the previous method. Patients with CTCs (epithelial + mixed + interstitial) showed an increased risk of postoperative death within the high CTCs group (P=0.002, Figure 3E), with a median survival of 30 months. As for patients with epithelial-type and mixed-type CTCs, no statistically significant difference was found for the risk of post operative death between the two groups (P=0.86, 0.18, respectively; Figure 3F,3G). In patients with interstitial-type CTCs, there was an increased risk of postoperative death (P=0.012, Figure 3H), with a median survival of 28 months.
The characteristics of c-Kit expression of CTCs in EC patients
In all 43 patients, preoperative CTCs were tested for c-Kit gene expression. The analyses showed there were no differences between age, gender, smoking history, drinking history, and family history in both of two groups of patients displaying c-Kit gene expression (P=0.32, 0.75, 0.45, 0.23 and 0.83, respectively). The rate of positive tests for c-Kit was 46% (20/43). The c-Kit expression was positive in 7 cases in stage I, in 2 cases in stage II, in 9 cases in stage III and in 2 cases in stage IV. There was no difference indicated between these two groups (Table 6).
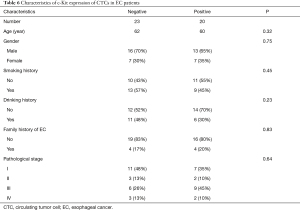
Full table
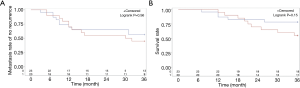
The relationship between c-Kit expression of CTCs and tumor recurrence/survival of EC patients
Kaplan-Meier survival analysis was performed in groups displaying both positive and negative c-Kit expression results and a log-rank test was applied for comparison between the two groups. The results showed that there was no significant difference in the risk of postoperative recurrence of tumors and metastasis between the two groups (P=0.56, Figure 4).
Discussion
EC is a common malignant tumor that has serious implications for the survival and life quality of patients. Although a 10% increase in 5-year survival rate was seen from 2005
We classified CTCs into the 3 subtypes of epithelial, mixed and interstitial based on their pathological features. We found an increased risk of post-operative recurrence of tumors, metastasis, and death in the high CTC group. The detection rates of epithelial-, mixed- and interstitial-type CTCs was 53%, 63%, and 33%, respectively. These results brought to light two issues in traditional epithelial-CTC detection surrounding the merging of the epithelial type and mixed type together and missing the interstitial-type CTCs. Further analysis confirmed that the risk of postoperative recurrence/metastasis and death were increased in patients with CTCs >0 compared with the group of interstitial CTCs =0.
CanPatrol® technology was applied in CTC detection of several malignancies including EC, breast cancer, prostate cancer, colorectal cancer, gastric cancer, to name a few. It has been widely accepted that the CanPatrol® method does not only classify the subtypes and improve the detection rate of CTCs, but is also associated with the prognosis of patients with interstitial-type CTCs (13-17). Chen et al. utilised the CanPatrol® method to study the relationship between CTCs and clinical stages of EC, finding that interstitial CTCs were related to the pathological stage of EC, consistent with our study. Additionally, the number of interstitial CTCs were associated with neoadjuvant chemotherapy of EC, but no prognostic analysis was conducted (18).
Our study highlighted the necessity of CTC detection in EC. A meta-analysis conducted by Li et al. demonstrated that CTCs could be considered as effective indicators in evaluating the efficacy of chemotherapy and monitoring tumor recurrence in EC patients (19). Hou et al. reported that disease-free survival (DFS) and overall survival (OS) both decreased in patients where CTCs were detected, compared with EC patients where no CTCs were detected (20). Interestingly, the rate of patients testing positive for CTCs was high in patients with stage I EC after operation (16/18). We hypothesized possible explanations as follows: (I) all EC patients in stage I belonged to stage IB; (II) in CTC positive cases, there were only 3 cases where CTCs >2 and 1 case where interstitial CTCs >0; (III) in regards to prognosis, there were 2 EC patients at stage I with recurrent tumors/metastasis and death within 3 years, where 2 cases displayed more than 2 CTCs and 1 case of interstitial type had CTCs >0. CTC test may be used as an early screening method for EC in the future. Further clinical trials with larger sample sizes should be conducted to confirm the usefulness of measuring CTCs in the early diagnosis of EC.
Few studies have reported on CTC changes before and after operation. Guo et al. (21) detected CTCs of EC patients before surgery using the CanPatrol® method, showing postoperative CTC levels were generally higher than levels during the operation, and the intraoperative CTCs were higher than they were pre-operation.
Furthermore, the current study indicated that postoperative complications may lead to an increase in CTCs. However, we found that while the postoperative number of CTCs in a few patients was elevated, overall the number of CTCs in patients were reduced post-operation. The reasons for this phenomenon may have been a result of the different surgical approaches undertaken. It has been reported minimally invasive esophagectomy helped to reduce the survival rate of tumor cells in peripheral blood at the early period of postoperation, and dynamic monitoring CTC level could be used to evaluate the prognosis of EC patients.
Few studies have reported the c-Kit expression in CTCs. In this study, the c-Kit expression of preoperative CTCs was detected in patients with EC by RNA-ISH. c-Kit expression was detected in CTCs of patients with malignant melanoma and prostate cancers (22,23). No other study has reported c-Kit expression in EC patients. In this study, the positive rate of c-Kit expression in CTCs was 46%, but no significant correlation between c-KIT expression and prognosis of EC patients was found. This result is in line with the study published by Shang and Boone et al. (24,25).
Limitations within this study were identified. Limited time and funds led to a small sample size which may have introduced bias into our results. The frequency of CTC detection was relatively low and reexamination of CTCs was not repeated during follow-ups. Therefore, we were not able to conclude that a change in CTC number could predict tumor recurrence/metastasis and long-term survival of patients. Another limitation of the study involved the analysis of only the c-Kit genes, whereas screening multiple genes at the same time could increase the probability of positive results.
In summary, the specific role of CTCs from peripheral blood in the clinical diagnosis, treatment, and disease progression of patients with EC requires further investigation. In future studies, a greater sample size and longer follow-up periods will be used to explore additional target genes involved in EC occurrence and progression, and to perform clinical trials for immunotherapy in EC. Additionally, single-cell sequencing technology could be useful in improving prospects for CTC detection.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-409
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jgo-21-409
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-409). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Ethical Committee of PLA General Hospital (No. 2021-147-03) and written informed consent was obtained from all patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Short MW, Burgers KG, Fry VT. Esophageal Cancer. Am Fam Physician 2017;95:22-8. [PubMed]
- Kulemann B, Liss AS, Warshaw AL, et al. KRAS mutations in pancreatic circulating tumor cells: a pilot study. Tumour Biol 2016;37:7547-54. [Crossref] [PubMed]
- Bidard FC, Proudhon C, Pierga JY. Circulating tumor cells in breast cancer. Mol Oncol 2016;10:418-30. [Crossref] [PubMed]
- Nanduri LK, Hissa B, Weitz J, et al. The prognostic role of circulating tumor cells in colorectal cancer. Expert Rev Anticancer Ther 2019;19:1077-88. [Crossref] [PubMed]
- Thanh Huong P, Gurshaney S, Thanh Binh N, et al. Emerging Role of Circulating Tumor Cells in Gastric Cancer. Cancers (Basel) 2020;12:695. [Crossref] [PubMed]
- Maly V, Maly O, Kolostova K, et al. Circulating Tumor Cells in Diagnosis and Treatment of Lung Cancer. In Vivo 2019;33:1027-37. [Crossref] [PubMed]
- Hoeppner J, Kulemann B. Circulating Tumor Cells in Esophageal Cancer. Oncol Res Treat 2017;40:417-22. [Crossref] [PubMed]
- Kang W, Zhu C, Yu J, et al. KIT gene mutations in gastrointestinal stromal tumor. Front Biosci (Landmark Ed) 2015;20:919-26. [Crossref] [PubMed]
- Wilcock A, Bahri R, Bulfone-Paus S, et al. Mast cell disorders: From infancy to maturity. Allergy 2019;74:53-63. [Crossref] [PubMed]
- Woodman SE, Davies MA. Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol 2010;80:568-74. [Crossref] [PubMed]
- Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. [Crossref] [PubMed]
- Zhang S, Wu T, Peng X, et al. Mesenchymal phenotype of circulating tumor cells is associated with distant metastasis in breast cancer patients. Cancer Manag Res 2017;9:691-700. [Crossref] [PubMed]
- Yang YJ, Kong YY, Li GX, et al. Phenotypes of circulating tumour cells predict time to castration resistance in metastatic castration-sensitive prostate cancer. BJU Int 2019;124:258-67. [Crossref] [PubMed]
- Wu F, Zhu J, Mao Y, et al. Associations between the Epithelial-Mesenchymal Transition Phenotypes of Circulating Tumor Cells and the Clinicopathological Features of Patients with Colorectal Cancer. Dis Markers 2017;2017:9474532 [Crossref] [PubMed]
- Liu DG, Xue L, Li J, et al. Epithelial-mesenchymal transition and GALC expression of circulating tumor cells indicate metastasis and poor prognosis in non-small cell lung cancer. Cancer Biomark 2018;22:417-26. [Crossref] [PubMed]
- Cheng B, Tong G, Wu X, et al. Enumeration And Characterization Of Circulating Tumor Cells And Its Application In Advanced Gastric Cancer. Onco Targets Ther 2019;12:7887-96. [Crossref] [PubMed]
- Chen W, Li Y, Yuan D, et al. Practical value of identifying circulating tumor cells to evaluate esophageal squamous cell carcinoma staging and treatment efficacy. Thorac Cancer 2018;9:956-66. [Crossref] [PubMed]
- Li Y, Wu G, Yang W, et al. Prognostic value of circulating tumor cells detected with the CellSearch system in esophageal cancer patients: a systematic review and meta-analysis. BMC Cancer 2020;20:581. [Crossref] [PubMed]
- Hou J, Zou K, Yang C, et al. Clinicopathological and prognostic significance of circulating tumor cells in patients with esophageal cancer: a meta-analysis. Onco Targets Ther 2018;11:8053-61. [Crossref] [PubMed]
- Guo X, Wu YZ, Jia LF, et al. Effects of minimally invasive versus open esophagectomy on circulating tumor cells in patients with esophageal cancer. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:318-23. [PubMed]
- Sakaizawa K, Goto Y, Kiniwa Y, et al. Mutation analysis of BRAF and KIT in circulating melanoma cells at the single cell level. Br J Cancer 2012;106:939-46. [Crossref] [PubMed]
- Russo GI, Bier S, Hennenlotter J, et al. Expression of tumour progression-associated genes in circulating tumour cells of patients at different stages of prostate cancer. BJU Int 2018;122:152-9. [Crossref] [PubMed]
- Shang L, Liu HJ, Hao JJ, et al. A panel of overexpressed proteins for prognosis in esophageal squamous cell carcinoma. PLoS One 2014;9:e111045 [Crossref] [PubMed]
- Boone J, van Hillegersberg R, Offerhaus GJ, et al. Targets for molecular therapy in esophageal squamous cell carcinoma: an immunohistochemical analysis. Dis Esophagus 2009;22:496-504. [Crossref] [PubMed]
(English Language Editors: B. Maizey and J. Chapnick)


