Positron emission tomography for initial staging of esophageal cancer among medicare beneficiaries
Introduction
Healthcare delivery in the United States (US) is increasingly focused on optimizing the value of care. Value—defined broadly as health benefits divided by costs—is not easily measured, but healthcare interventions without benefits certainly have no value. A well-recognized problem in cancer care is the lack of evidence linking the use of diagnostic tests to improved patient outcomes. This uncertainty is particularly problematic with advanced imaging modalities, such as positron emission tomography (PET), because these tests are expensive. For instance, five randomized trials demonstrated that PET use leads to improved staging accuracy among lung cancer patients (1-5). However, better staging accuracy in these trials was never demonstrated to lead to better survival. Three of the trials measured survival as a secondary endpoint but none showed survival improvements attributable to PET (1,3,5). Nonetheless, the benefit of PET, measured in terms of staging accuracy, has been extrapolated to other disease sites, including esophageal cancer—a deadly malignancy with a rising incidence in the US (6-8).
The role of PET in the initial staging of esophageal cancer patients is to identify individuals with occult metastases not detected by physical examination and computed tomography (CT) (9). Identification of metastases is important because it allows the patient to avoid the morbidity of interventions that have no known efficacy for stage IV disease, including esophagectomy and/or multi-modality therapy (10). However, recent studies demonstrating a low rate (8–20%) of occult metastases detected by PET, and a highly variable rate of false-positive results (0–60%) raises questions about the benefit of PET in esophageal cancer (9,11-19). Despite Medicare approval for PET reimbursement in 2001 (20), its effectiveness among esophageal cancer patients has never been evaluated at the population-level. Reimbursement of PET by Medicare would be expected to result in a rapid rise in the utilization of PET. If effective at diagnosing occult metastases, one would also expect the prevalence of stage IV disease to increase coincidently. The primary aim of this investigation was to test these hypotheses using the Surveillance, Epidemiology, and End-Results (SEER)-Medicare database.
Methods
The University of Washington Institutional Review Board approved this retrospective cohort study of patients, diagnosed with esophageal cancer between 1997 and 2009 using the SEER-Medicare database. SEER is a tumor registry—drawn from 18 population-based cancer registries representing 28% of the US population (21)—and is linked to Medicare claims data. The linked database has been validated and is generalizable (22). Although SEER began capturing incident cases of pathologically confirmed esophageal cancer in 1992, the period of observation was limited to 1997 to 2009 to ensure a uniform staging classification for esophageal cancer throughout the study. The definition of stage IV disease did not change between the 5th and 6th editions of the American Joint Committee on Cancer (AJCC) staging manual, and therefore the definition of stage IV disease was constant throughout the study.
Elderly Medicare beneficiaries (age >65) diagnosed with primary esophageal cancer [International Classification of Diseases for Oncology, Third Edition (ICD-O-3), codes 15.0–15.5, 15.8–15.9, Appendix 1] were considered potentially eligible for study (n=23,458). In order to ensure the completeness of claims data, patients without continuous enrollment in both Medicare parts A and B and/or concurrent enrollment in a health maintenance organization (HMO) the year prior to and four months after diagnosis were excluded (n=8,506). Patients were sequentially excluded for the following additional reasons: diagnosis of another malignancy in the 4 months after diagnosis of esophageal cancer (n=372); diagnosis at the time of autopsy or death or based on hospice records (n=365); and missing covariate information (n=1,345). The remaining 12,870 patients were included in our study.
Demographic variables were obtained from SEER records and included age, sex, race, marital status, and socioeconomic variables. A modified Charlson comorbidity index was calculated using claims in the year prior to diagnosis within the Physician/Carrier and Outpatient files (23). Household income was derived using the median household income per zip code from the 1,990 census bureau survey for those patients diagnosed between 1997 and 1999 and using 2,000 census bureau for those diagnosed between 2000 and 2009. SEER also measures cancer variables. Prior malignancy denotes any prior history of cancer other than esophageal cancer. Histology of esophageal cancer was classified as adenocarcinoma, squamous cell carcinoma or other by using the ICD-O-3 code for the SEER diagnosis based on the AJCC manual. Tumor characteristics, including T, N, and M stage, were obtained from SEER records. Stage is recorded by SEER abstractors based on the highest level of available information within four months of diagnosis.
Healthcare Common Procedure Coding System (HCPCS) codes were used to ascertain PET use (Appendix 1). PET was approved for diagnosis and staging of esophageal cancer by Medicare in 2001 (20). For descriptive purposes patients were grouped into the time period before PET coverage [1997–2000] and after PET coverage [2001–2009]. Dates of death were identified using the Medicare Enrollment Database with information available through December 31, 2010. One-year overall survival rates are described, with survival times measured from the date of diagnosis.
Baseline patient characteristics, stage distribution, claims for treatment modalities, and survival were compared between the pre-PET [1997–2000] and post-PET [2001–2009] claim eras using Chi-squared tests binary and categorical variables and the Wilcoxon rank-sum (Mann-Whitney) test for continuous variables that were not normally distributed. Overall trends in PET use, stage distribution, therapy, and survival were plotted by year of diagnosis from 1997 through 2009. Because our expected outcome prevalence was high (>10%), and because odds ratios tend to magnify the magnitude of associations found in studies with non-rare outcomes (24), we report trends in use of PET and prevalence of stage IV disease as relative risks (RR). To estimate RR, we used Poisson regression with robust sandwich-style variance estimators using logarithm as the natural link function under the generalized linear model framework. The estimates of these models are believed to be more appropriate for estimating common outcomes and less susceptible to influence of outlier data (24,25). Variables used for adjustment included age, gender, race, income, education, marital status, prior malignancy, comorbidity index, histology, and SEER region. All models were clustered on SEER region. STATA version 13.0 (Statacorp, College Station, Texas) was used for all statistical analyses.
Results
Between 1997 and 2009, 12,870 patients (mean age 76.8±7 years, 71% male, 87% white) newly diagnosed with esophageal cancer met eligibility criteria for this study. SEER accrues additional tumor registries over time, which accounts for the substantially larger proportion (79%) of patients included in the database in the post-PET era. Nonetheless, 2,652 patients from the pre-PET era were available for analysis (Table 1). Compared to subjects in the pre-PET era, patients from the post-PET era were older and had more comorbid conditions. As expected, there was a higher prevalence of adenocarcinoma in the more contemporary cohort (55% vs. 47%, P<0.001). There was also a markedly higher proportion of stage I patients in the post-PET era compared to the pre-PET era (14% vs. 3%, P<0.001). The prevalence of patients with stage IV disease was higher (25% vs. 21%, P<0.001), and the proportion of patients with stage-not-recorded (NR) was significantly lower (22% vs. 37%, P<0.001). One-year overall survival in the post-PET era was slightly higher (41% vs. 37% in the pre-PET era, P<0.001).

Full table
The use of PET increased more than fifteen-fold (2.7% before 2001 to 44% in 2009) over the years subsequent to Medicare’s approval for reimbursement (Figure 1). Even after adjustment for changing patient characteristics over time, a large and increasing trend in the use of PET was evident (RR =1.17; 95% CI, 1.14–1.20, adjusted P trend <0.001) (Table 2). Over the entire study period (1997 to 2009) the proportions of patients with stage 0–III and Stage IV disease also increased over time (adjusted P trend <0.001 for both). This steady rise in both categories of stage 0–III and stage IV disease coincided with a large drop in the proportion of patients with stage NR (adjusted P trend <0.001). Because the prevalence of stage IV disease was increasing prior to the approval of PET in 2001, we performed an analysis to determine if rates of change were different between the pre- and post-PET approval eras. In the adjusted analysis taking into account changing patient and tumor characteristics during this time, we found no significant differences (pre-PET RR =1.06, 95% CI, 1.00–1.13, P trend =0.049 vs. post-PET RR =1.02; 95% CI, 1.02–1.04, P trend <0.001).
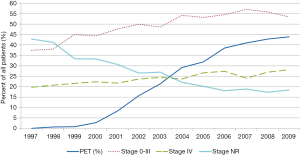

Full table
Discussion
The principal role of PET in the initial staging of esophageal cancer is detection of occult metastases. The benefit of identifying occult metastases is avoiding the risks of curative-intent therapy in patients who will not benefit from this type of treatment. We sought to indirectly evaluate the effectiveness of PET in detecting occult metastatic disease at the population-level by looking for an increase in the prevalence of stage IV disease over time coinciding with anticipated rapid adoption of PET after 2001. As expected, the use of PET increased over fifteen-fold over the nine years after Medicare approved reimbursement of this advanced imaging modality for esophageal cancer staging. Corresponding to the rise in PET use was an increase in the prevalence of stage IV disease over time. However, the prevalence of stage IV disease was increasing at a similar rate prior to and after the adoption of PET. These findings do not support the hypothesis that PET meaningfully changes the detection of occult stage IV disease in esophageal cancer at the population-level, and draw attention to the value of PET for initial staging of esophageal cancer.
The rise in prevalence of stage IV disease observed in this study is more likely an artifact of measurement than a consequence of increasing PET use. Specifically, better documentation and recording of cancer stage within the SEER registry over time most likely explains increasing rates of stage IV disease (and stage 0-III disease for that matter). Investigators familiar with the SEER database are aware of the significant proportion of patients with missing stage information, and indeed, including patients with missing stage information in our study revealed a marked decline (by ~40%) in the proportion of patients with missing stage data over time. At the same time, we found an increase in the frequency of all stages of disease. This observation is most likely explained by better staging documentation over time rather than a true change in the distribution of esophageal cancer stage. Besides improved documentation, the only other clinically plausible explanation for an increase in the prevalence of stage IV disease over the study period is the rapid adoption of PET. However, the finding that there was no difference in the increasing prevalence of stage IV disease between the pre- and post-PET eras undermines this claim.
Several reasons may explain why we did not find compelling evidence of a benefit of PET at detecting metastatic disease at the population-level. One explanation is that the purported benefits of PET are too small to be observed at the population-level. Single-institution investigations report that the frequency of true occult metastatic disease detected by PET is low, ranging from 8–20% (9,11-19). Our study shows that despite the rapid adoption in PET use, less than half of patients underwent PET in 2009. This combination of low PET utilization and infrequent metastatic disease detection may explain the lack of a population-level “effect” of PET. Another possibility is that PET has diminishing benefits over time. For instance, improving resolution of CT imaging for distant metastases may be contributing to an apparent lack of population-level impact. A third reason for the lack of apparent benefit of PET over time is increasing adoption of esophageal cancer screening in the community-at-large. The proportion of patients with stage I disease increased markedly over the study consistent with efforts to screen high-risk patients with Barrett’s esophagus or hereditary syndromes (26). Earlier-detection of disease would lead to fewer patients who could benefit from PET’s ability to detect occult metastatic disease. To the extent that the increase in stage I in this study is attributable to greater screening, a proportional decrease in stage IV would be expected. More screening and early-detection may have countered a rise in stage IV stage attributable to PET, but the primary argument against this explanation is the finding that the rate of increase in stage IV did not change in the pre- and post-PET eras.
This study has several key limitations. We were unable to directly evaluate the benefit of PET because of an inability to access clinically granular information using this dataset. For example, a direct measure of the benefit of PET would be the frequency of patients identified to have occult metastatic disease missed by physical examination and CT (i.e., upstaging attributable to PET). Another direct measure would be the frequency of curative-intent treatments avoided among patients with stage IV disease. Because of our indirect measurement of benefit (i.e., prevalence of stage IV), we are unable to tease out the relative contributions (if any) of PET, measurement phenomena (e.g., missing stage information), and increased screening in the community-at-large to the rising prevalence of stage IV disease. Lack of clinically rich data also precluded studying other potentially valuable applications of PET, such as re-staging and/or response to therapy (27,28). Another important limitation of this study is generalizability. It is unclear whether temporal trends observed in this study also exist in the non-elderly and/or commercially insured population of esophageal cancer patients. The Cancer Research Network may be one way to study these trends in the future among non-elderly patients enrolled in integrated health systems (29). There are no other databases that collect longitudinal information on cancer patients in the inpatient and outpatient setting for the non-elderly across a variety of health plans. Importantly, however, our study is representative of a majority of Americans with esophageal cancer given that 60% of new diagnoses of esophageal cancer occur in patients older than 65 (8).
Our study compliments the work of others drawing attention to the opportunity to increase the value of PET. Although the routine use of PET for initial staging of esophageal cancer remains the standard of care (10,30), a growing number of investigators have suggested selective use of PET as an alternative approach to staging esophageal cancer (31,32). The basis for this proposal is the low rate at which PET identifies occult metastatic disease above and beyond physical examination and CT. However, there is no universally accepted threshold for what constitutes “a low rate” leading to the converse and prevailing conclusion that PET should be used routinely (10,16). From a patient’s perspective, even a 5% chance to avoid ineffective curative-intent treatments for stage IV disease would seem to be of great value. Nonetheless, a recent investigation highlights the need to balance the desire to “rule out distant disease using any means necessary” against the high rate of false-positive PET results that can lead to unnecessary procedures, potential treatment delays, and higher costs (32). Our study contributes to the broader dialogue by providing the first population-based assessment of the impact of PET. Limitations notwithstanding, we found no compelling evidence of a meaningful impact of PET on a population of esophageal cancer patients. While these results do not supersede the individual perspective, they do offer another perspective by which to consider the value of advanced imaging in an era of increasingly limited healthcare resources. One approach to balancing the needs of a population and individuals is through risk-based strategies. Prediction models are increasingly used in cancer care to estimate the chance of a particular event for the purpose of improving medical decision-making (33). The estimated probability of metastatic disease could be used to stratify patients into high- and low-risk groups. High-risk patients would undergo PET whereas low-risk patients would proceed with curative-intent treatment. This selective approach allows for variability in the use of PET based on patient-level risk-factors while providing structure for an invariant approach to esophageal cancer staging at the provider-level. By facilitating personalized cancer care and responsible stewardship of limited resources, a risk-based selective approach to PET utilization would likely increase its value.
Acknowledgements
The authors would like to acknowledge Hao He, PhD for assistance with analytic dataset design.
Funding: Research reported in this publication was supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T32DK070555 and the National Cancer Institute under Award Number CA009168. Dr. Farjah received support as a Cancer Research Network Scholar (CRN4: Cancer Research Resources & Collaboration in Integrated Health Care Systems, grant number U24 CA171524).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Cancer Institute or the Cancer Research Network.
Supplementary
Appendix 1 Codes used to define claims related to esophageal cancer and PET scan use
Esophageal cancer: International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes
15.0 = “C15.0-Cervical esophagus”;
15.1 = “C15.1-Thoracic esophagus”;
15.2 = “C15.2-Abdominal esophagus”;
15.3 = “C15.3-Upper third of esophagus”;
15.4 = “C15.4-Middle third of esophagus”;
15.5 = “C15.5-Lower third of esophagus”;
15.8 = “C15.8-Overlapping lesion of esophagus”;
15.9 = “C15.9-Esophagus, NOS”.
PET scan: Healthcare Common Procedure Coding System (HCPCS) codes
G0125, G0126, G0163, G0164, G0165, G0235, G0252, G0253, G0254, G0296, G0330, G0331, 78810–78816, G0210-G0228, G0231-G0234, G0213-G0215, and G0226-G0228.
References
- Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 2009;361:32-9. [Crossref] [PubMed]
- Herder GJ, Kramer H, Hoekstra OS, et al. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: a Dutch cooperative randomized study. J Clin Oncol 2006;24:1800-6. [Crossref] [PubMed]
- Maziak DE, Darling GE, Inculet RI, et al. Positron emission tomography in staging early lung cancer: a randomized trial. Ann Intern Med 2009;151:221-8, W-48.
- van Tinteren H, Hoekstra OS, Smit EF, et al. Toward less futile surgery in non-small cell lung cancer? A randomized clinical trial to evaluate the cost-effectiveness of positron emission tomography. Control Clin Trials 2001;22:89-98. [Crossref] [PubMed]
- Viney RC, Boyer MJ, King MT, et al. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol 2004;22:2357-62. [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005;97:142-6. [Crossref] [PubMed]
- SEER cancer statistics factsheets: Esophageal cancer. Available online: . Updated 2014.http://seer.cancer.gov/statfacts/html/esoph.html
- Heeren PA, Jager PL, Bongaerts F, et al. Detection of distant metastases in esophageal cancer with (18)F-FDG PET. J Nucl Med 2004;45:980-7. [PubMed]
- Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw 2011;9:830-87. [PubMed]
- Meyers BF, Downey RJ, Decker PA, et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. J Thorac Cardiovasc Surg 2007;133:738-45. [Crossref] [PubMed]
- Noble F, Bailey D; SWCIS Upper Gastrointestinal Tumour Panel, et al. Impact of integrated PET/CT in the staging of oesophageal cancer: a UK population-based cohort study. Clin Radiol 2009;64:699-705. [Crossref] [PubMed]
- Salahudeen HM, Balan A, Naik K, et al. Impact of the introduction of integrated PET-CT into the preoperative staging pathway of patients with potentially operable oesophageal carcinoma. Clin Radiol 2008;63:765-73. [Crossref] [PubMed]
- Gillies RS, Middleton MR, Maynard ND, et al. Additional benefit of 18F-fluorodeoxyglucose integrated positron emission tomography/computed tomography in the staging of oesophageal cancer. Eur Radiol 2011;21:274-80. [Crossref] [PubMed]
- You JJ, Wong RK, Darling G, et al. Clinical utility of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the staging of patients with potentially resectable esophageal cancer. J Thorac Oncol 2013;8:1563-9. [Crossref] [PubMed]
- Purandare NC, Pramesh CS, Karimundackal G, et al. Incremental value of 18F-FDG PET/CT in therapeutic decision-making of potentially curable esophageal adenocarcinoma. Nucl Med Commun 2014;35:864-9. [PubMed]
- Kato H, Miyazaki T, Nakajima M, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 2005;103:148-56. [Crossref] [PubMed]
- Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg 1999;68:1133-6; discussion 1136-7. [PubMed]
- Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202-10. [PubMed]
- CMS Manual System. Pub 100-03 Medicare National Coverage Determinations. Available online: https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R31NCD.pdf
- National Cancer Institute. Surveillance, epidemiology, and end-results program. Available online: . Updated 2015. Accessed 06/06, 2015.http://seer.cancer.gov/about/overview.html
- Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV-3-18. [Crossref] [PubMed]
- Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258-67. [Crossref] [PubMed]
- Chen W, Shi J, Qian L, et al. Comparison of robustness to outliers between robust poisson models and log-binomial models when estimating relative risks for common binary outcomes: a simulation study. BMC Med Res Methodol 2014;14:82. [Crossref] [PubMed]
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. [Crossref] [PubMed]
- Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc 2006;63:570-80. [Crossref] [PubMed]
- Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med 2006;354:496-507. [Crossref] [PubMed]
- Smyth EC, Shah MA. Role of 18F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies. World J Gastroenterol 2011;17:5059-74. [Crossref] [PubMed]
- National Cancer Institute. The cancer research network. Available online: . Updated 2015. Accessed 06/06, 2015.http://crn.cancer.gov/
- Varghese TK Jr, Hofstetter WL, Rizk NP, et al. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg 2013;96:346-56. [Crossref] [PubMed]
- Bunting DM, Lai WW, Berrisford RG, et al. Positron emission tomography-computed tomography in oesophageal cancer staging: a tailored approach. World J Surg 2015;39:1000-7. [Crossref] [PubMed]
- Cuellar SL, Carter BW, Macapinlac HA, et al. Clinical staging of patients with early esophageal adenocarcinoma: does FDG-PET/CT have a role? J Thorac Oncol 2014;9:1202-6. [Crossref] [PubMed]
- Vickers AJ. Prediction models in cancer care. CA Cancer J Clin 2011;61:315-26. [PubMed]


