Masquerade without a mass: an unusual cause of severe acute pancreatitis
Introduction
Extra-nodal involvement of non-Hodgkin’s lymphoma occurs in more than 50% of patients but pancreatic lymphoma is uncommon. We present a rare case of pancreatitis caused by infiltrating large B-cell lymphoma, a diagnosis made difficult by its unusual presentation.
Case report
A 74-year-old woman with a past medical history notable only for resolved pneumonia one month prior, presented with two weeks of nausea, vomiting and epigastric pain radiating to her back. Review of systems was positive for generalized weakness and a 14-pound weight loss over the previous three weeks. She denied alcohol intake or recent trauma. She was evaluated in urgent care and diagnosed with acute pancreatitis. After failed outpatient management, she was admitted due to an inability to maintain adequate oral intake.
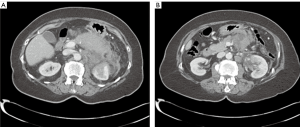
Vital signs included temperature 37 °C, blood pressure 169/71 mmHg, pulse 110 beats/min, respiratory rate 18 breaths/min, and oxygen saturation 96% on room air. Physical examination was remarkable for epigastric tenderness without guarding or palpable abdominal mass. Initial laboratory studies demonstrated an elevated lipase at >3,000 U/L (reference, 73-393 U/L), amylase 268 U/L (reference, 30-110 U/L), and white blood count (WBC) 11.8×109 /L. Her liver enzymes were normal, as was her triglyceride level at 133 mg/dL. Abdominal ultrasound showed no evidence of gallstones or cholecystitis. Computed tomography (CT) of the abdomen and pelvis revealed severe pancreatitis without an obvious focal lesion (Figure 1A), as well as developing pseudocysts and nonspecific upper abdominal and right retrocrural lymphadenopathy (Figure 1B).
The patient improved with conservative management and was discharged home; however, she was re-admitted 9 days later with intractable nausea and continued weight loss. The patient’s second admission was complicated by severe malnutrition with hypoalbuminemia of 1.0 g/dL (reference, 3.3-5.0 g/dL), anasarca and a rising WBC to 20.0×109/L without clear evidence of infection. Surgeons were consulted for aspiration and/or drainage of the pseudocysts but felt their small size made infection unlikely. Due to worsening abdominal pain, magnetic resonance cholangiopancreatography (MRCP) was attempted but not tolerated due to severe claustrophobia. Repeat CT demonstrated worsening acute pancreatitis, and a chest X-ray demonstrated a large left pleural effusion, in the setting of an increased oxygen requirement. Chest CT demonstrated a new cavitary lesion; however, bronchoscopic lavage was negative for acid-fast bacteria, bacterial and fungal growth. Bedside thoracentesis was performed, and repeated when fluid rapidly re-accumulated, demonstrating an exudative effusion. Fluid cytology was negative for malignancy. However, large volume fluid analysis from a subsequently placed chest tube demonstrated “atypical cells present, suspicious for malignancy.”
A repeat CT scan performed on day 21 of the patient’s second admission was “suspicious for an infiltrative process causing secondary pancreatitis.” Cancer antigen CA 19-9 was normal, but lactate dehydrogenase (LDH) was elevated at 729 U/L (reference, 84-246 U/L). Endoscopic ultrasound (EUS) with biopsy of abnormal pancreatic tissue or adenopathy was planned; however, the patient became unstable with a blood pressure of 70/40 mmHg and oxygen saturation of 70%. She was transferred to the ICU for hemodynamic shock and respiratory failure. Cytology from a 4th pleural fluid sample showed atypical large cells now suspicious for large cell lymphoma. The family chose a do-not-resuscitate status, and the patient expired.
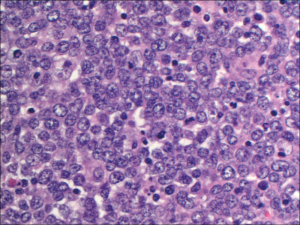
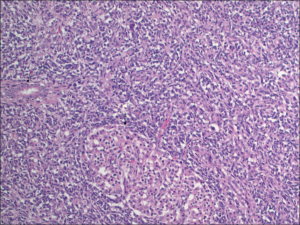
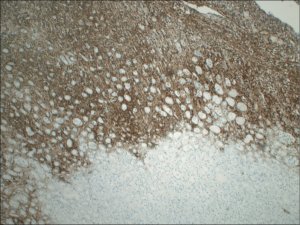
An autopsy revealed diffuse large B-cell lymphoma involving the pancreas, spleen, left kidney, retroperitoneum, and mesentery with enlarged periaortic lymph nodes. Figure 2 demonstrates a high power image of peripancreatic tissue with well preserved lymphomatous infiltrate, characterized by large cells with round nuclei and occasional prominent nucleoli. An intermediate magnification of pancreas tissue demonstrates diffuse infiltration of lymphoma with near effacement of normal pancreatic architecture (Figure 3). A kidney tissue section stained with antibody directed against CD20, highlighting the malignant cells, demonstrates disrupted renal tissue with lymphoma infiltrating between the tubules (Figure 4).
Discussion
Extra-nodal involvement of non-Hodgkin’s lymphoma occurs in more than 50% of patients (1,2). The most frequent site is the gastrointestinal tract, especially the stomach and small intestine (1-3). In contrast, pancreatic lymphoma is uncommon: less than 0.5% of pancreatic tumors are of lymphomatous origin, and only 0.2-2% of patients with non-Hodgkin’s lymphoma have pancreatic involvement at presentation (1-5).
Most cases of pancreatic lymphoma are of the diffuse large B-cell type (1). The diagnosis of pancreatic lymphoma may be difficult, as symptoms, laboratory studies and imaging are often nonspecific (1,4). LDH can be elevated in 50% of cases and tumor marker CA 19-9 may occasionally be elevated (1). The most common presentation of pancreatic lymphoma is abdominal pain [83% of cases (4)], as well as weight loss, nausea and vomiting (1). Typical B-symptoms of lymphoma, such as fever and night sweats, are uncommon (1).
Pancreatic lymphoma is itself unusual, but pancreatic lymphoma presenting as acute pancreatitis is rare. To our knowledge, only twelve other cases of pancreatic B-cell lymphoma presenting as acute pancreatitis have been described (1-5). In contrast, pancreatic adenocarcinoma associated with pancreatitis is a well-documented phenomenon with up to 14% of cases of adenocarcinoma of the pancreas presenting as acute pancreatitis (6). The proposed mechanisms of pancreatic lymphoma presenting as pancreatitis include “ductal obstruction, ductal rupture with direct parenchymal tumor invasion, and ischemia secondary to vascular occlusion by tumor” (7). There are two described patterns of pancreatic involvement: (I) a discrete, well circumscribed tumor; and (II) a diffuse infiltrating process that may mimic findings of acute pancreatitis on imaging, such as seen in our case (8). Interestingly, a review in the American Journal of Radiology stated that the latter pattern “never show[s] the typical clinical signs of acute pancreatitis even if the serum amylase is elevated” (8), but this statement is contradicted by the findings of our case. Notably, all prior case reports of pancreatic lymphoma presenting as acute pancreatitis appear to be of the well-circumscribed pattern, lending themselves to easy diagnosis by biopsy of the discrete mass. In contrast, a diffuse infiltration of the pancreas makes the diagnosis more difficult due to its similarity to pancreatitis on imaging.
High grade pancreatic lymphoma responds well to chemotherapy (2). Standard treatment would include 6-8 cycles of rituximab, cyclophosphamide, hydoxydaunorubicin, vincristine, prednisone (R-CHOP) (5,8) with a cure rate of approximately 30-40%, depending on stage. If a patient is unable to tolerate chemotherapy, treatment with radiation or steroids can be considered until his or her clinical status improves. In our case, the diagnosis was made at autopsy. This is unfortunate given that her disease was treatable, and potentially curable. In retrospect, it was exceedingly difficult to distinguish severe pancreatitis from a diffusely infiltrating malignancy, as both imaging and initial pleural cytologies were nonspecific. The final cytology showing a clonal large B-cell population may have been sufficient for diagnosis and treatment, but was not available early enough to change the course of her disease.
What might have led to an earlier diagnosis? Lack of clinical improvement with standard management should increase clinical suspicion for rare diseases and perhaps suggest use of adjunctive diagnostic studies. While the surgeons were reluctant to perform a biopsy, due to the risk of inducing worsening pancreatitis or fistulization, earlier tissue sampling by EUS or interventional radiology CT-guidance might have led to the correct diagnosis. In this case, biopsies of the lymphadenopathy or ill-defined involvement of the left kidney would have likely been of higher yield than biopsy of the pancreas, since diagnosing pancreatic malignancy is limited by the presence of acute or chronic pancreatitis in the biopsy specimen (9). Positron emission tomography (PET) could have been performed once malignant cells were suspected in the pleural sample, to identify other hypermetabolic regions as potential targets for biopsy. Our patient received empiric methylprednisolone just prior to ICU admission, once lymphoma was suspected from the CT appearance and high LDH, but clinical instability precluded any further biopsy attempts. She unfortunately derived no clinical benefit.
This case demonstrates a diffuse infiltrating malignancy masquerading as typical acute pancreatitis and serves as a reminder to consider lymphoma or other tumors in the differential diagnosis of pancreatitis, after excluding the more typical causes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Federico E, Falconi M, Zuodar G, et al. B-cell lymphoma presenting as acute pancreatitis. Pancreatology 2011;11:553-6.
- Saif MW, Khubchandani S, Walczak M. Secondary pancreatic involvement by a diffuse large B-cell lymphoma presenting as acute pancreatitis. World J Gastroenterol 2007;13:4909-11.
- Lee MK, Jeon SW, Lee YD, et al. A case of primary pancreatic non-Hodgkin’s lymphoma. Korean J Intern Med 2006;21:123-6.
- Bernardeau M, Auroux J, Cavicchi M, et al. Secondary pancreatic involvement by diffuse large B-cell lymphoma presenting as acute pancreatitis: treatment and outcome. Pancreatology 2002;2:427-30.
- Sugishita H, Watanabe Y, Yamamoto Y, et al. Primary Pancreatic Lymphoma: The Role of Surgical Treatment. Case Rep Gastroenterol 2010;4:104-110.
- Thomas PC, Nash GF, Aldridge MC. Pancreatic acinar cell carcinoma presenting as acute pancreatitis. HPB (Oxford) 2003;5:111-3.
- Safadi R, Or R, Bar Ziv J, et al. Lymphoma-associated pancreatitis as a presenting manifestation of immunoblastic lymphoma. Leuk Lymphoma 1994;12:317-9.
- Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol 2000;174:671-5.
- Eloubeidi MA, Tamhane AR, Buxbaum JL. Unusual, metastatic, or neuroendocrine tumor of the pancreas: a diagnosis with endoscopic ultrasound-guided fine-needle aspiration and immunohistochemistry. Saudi J Gastroenterol 2012;18:99-105.


