Preoperative and surveillance MR imaging of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy
Introduction
The important role of surgical cytoreduction and heated intraperitoneal chemotherapy (HIPEC) in patients with peritoneal surface malignancies is well established (1-13). The PCI is the most widely validated and precise quantitative prognostic indicator (14-16). For patients undergoing CRS and HIPEC the PCI is one factor associated with determining whether a complete surgical cytoreduction can be achieved (17).
Preoperative MRI and CT of the abdomen and pelvis play an integral role in determining the extent of peritoneal and visceral disease in patients being considered for CRS and HIPEC for appendiceal, ovarian, colorectal, primary peritoneal, gastric, mesothelioma and other rare types of gastrointestinal disease involving the peritoneum (18-26). Careful patient selection based on preoperative imaging may prevent unnecessary surgeries in patients whose tumors are too extensive and cannot be adequately cytoreduced. Following CRS and HIPEC surveillance imaging combined with serial tumor markers are routinely used to detect recurrent tumor (27).
In this article we will discuss the technical issues surrounding peritoneal MR imaging, including patient preparation and MR scanning protocols. Image interpretation in the preoperative and surveillance setting will be discussed. Comparisons with alternative imaging tests such as CT and the clinical utility of MR imaging for preoperative assessment of peritoneal cancer index (PCI) and for surveillance of patients following CRS and HIPEC will be described.
Comparisons with other imaging tests
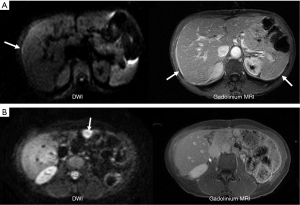
While CT is limited to assessing attenuation of X-rays, MR imaging uses multiple contrast mechanisms to improve its sensitivity for depicting small peritoneal tumors. Initial experience confirmed that peritoneal tumors show marked enhancement on images obtained 5 min after administration of gadolinium contrast material (28,29). The increased conspicuity of these enhancing peritoneal tumors improved detection of small and microscopic tumors that are often missed on CT scans (30,31) (Figure 1). The addition of diffusion imaging to the MRI tool chest further improves peritoneal tumor depiction. Diffusion-weighted (DW) MR images assess microscopic movement of water protons (32,33). Most tumors restrict water diffusion causing them to appear as high signal areas on diffusion images. In our experience the combination of diffusion weighted imaging (DWI) and delayed gadolinium-enhanced MR imaging is most accurate for detecting peritoneal tumors (34,35) (Figure 2).
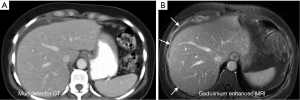
Multidetector CT is commonly used for preoperative imaging in patients undergoing surgical cytoreduction but is very limited in its ability to depict small peritoneal tumors. Coakley et al. (30) noted sensitivity of helical CT for peritoneal tumors < than 1cm was only 25-50% compared with 85-95% for all tumors. Low et al. (31) reported the sensitivity of gadolinium enhanced MR images for depicting peritoneal tumors at < than 1 cm was 85-90% compared to 22-33% for CT. The average sensitivity of MR for depicting peritoneal tumors of all sizes was 84% compared with 54% for CT. Klumpp et al. reported similar results with gadolinium-enhanced MRI demonstrating an 87% segment sensitivity and 88% accuracy for depicting peritoneal tumors compared to surgical findings (25).
In a multi-institutional study Esquivel et al found that the preoperative CT PCI score underestimated the extent of carcinomatosis in 33% of patients (36). The poor sensitivity of CT for detecting small peritoneal tumors limits its accuracy in determining a patient’s preoperative PCI score (36,37). There is also growing concern regarding the excessive radiation exposure that patients receive from repeated CT scans (38,39). The use of PETCT has been explored in patients with peritoneal carcinomatosis with improved results compared to CT alone (35,40). Our experience indicates that subtle small volume peritoneal tumors are not well depicted on PET.
Technical considerations and protocols for peritoneal MRI
Patient preparation
All patients are asked not to eat or drink for the four hours prior to their MR appointment. If rectal water is to be administered, patients self administer a Fleet’s enema prior to the examination.
Intraluminal contrast material
Water soluble intraluminal contrast material is administered to distend the stomach, small bowel, and colon. Collapsed bowel can mask subtle peritoneal tumors or inflammation involving the bowel serosa, mesentery, or adjacent peritoneum. Alternatively, non distended segments of small bowel can be mistaken for an abdominal mass. Adequate bowel distention is therefore an essential element in the peritoneal MR imaging protocol that improves the accuracy and confidence on image interpretation (31).
Water soluble contrast material is administered orally beginning 45 minutes before the start of the MR examination. Water soluble contrast materials are biphasic on MR images producing high intraluminal signal on T2-weighted images and low signal intensity on T1-weighted, and gadolinium-enhanced SGE images. Patients drink 1.0-1.5 liters of oral contrast material of sufficient volume to distend the small bowel and stomach. There are a number of different available oral contrast agents that can be used for MR imaging. While their use for MR imaging is off label they have proven to be safe and effective for bowel distention. These oral contrast agents are predominantly water with some other agents added to decrease absorption of the material through the small bowel wall. We currently use dilute barium sulfate suspension CT contrast material which is 98% water. E-Z-EM Readi-CAT2® (Bracco) has been well tolerated and effective in our practice when used to distend the small bowel for MR imaging. Fruit flavored versions of the oral contrast material are available which may improve patient compliance. Chilling the oral contrast material is also preferred by some patients. A bottle of this oral agent can also be administered at home to increase the transit and distension of the distal small bowel. Another oral agent that has been adopted for gastrointestinal MR imaging is VoLumen® Barium Sulfate Suspension, 0.1% w/v, 0.1% w/w, 450 mL (Bracco). VoLumen was designed as a negative CT intraluminal contrast agent and is composed of sorbitol, bean gum, and water. For the purposes of MR imaging it is predominantly water and appears as a biphasic intraluminal agent that is identical to dilute barium sulfate suspension products.
Distention of the rectum and colon can be accomplished with 1 L of tap water administered through a balloon tipped barium enema catheter. The balloon should be filled with water and not air to decrease the susceptibility artifact that the air would create. While rectal water is not an absolute requirement it can improve the depiction of subtle serosal and peritoneal tumor involving the colon and rectum. In other patients one may find that the colon is adequately distended with stool so that rectal water is not required.
Intravenous contrast agents
Intravenous gadolinium chelate is administered using a power injector at an injection rate of 2 cc per second through an angiocatheter. In the past we have used a double dose of intravenous gadolinium to increase the degree of enhancement of peritoneal tumors and inflammation. We currently use a single dose 0.1 mmol/kg of MultiHance® (gadobenate dimeglumine) (Bracco), which due to its higher relaxivity may show greater enhancement of peritoneal tumors. To our knowledge a comparison of Multihance and other gadolinium chelates for depicting peritoneal disease has not been performed.
Antiperistaltic agents
A medication should be administered to decrease bowel peristalsis on the gadolinium-enhanced images. The 3D FSPGR and 2D SGE images are sensitive to bowel motion and image quality is improved by administering an antiperistaltic pharmacologic agent. Available agents include Glucagon for injection (Eli Lilly and Company, Indianapolis, IN, USA) 1 mg administered intravenously at the time of gadolinium injection, Buscopan® (hyoscine-N-butylbromide), and Levsin® (hyoscyamine sulfate injection) 0.25 mg administered intravenously at the start of the examination. Package inserts should be carefully reviewed for all of these medications prior to their use to understand contraindications and potential drug interactions.
MR hardware—MR scanner and coils
1.5 or 3 T high field strength MR scanner should be used for imaging peritoneal tumors. High performance gradients (50 mT/m, 200 mT/m/sec) are advantageous for high quality DW imaging but are not absolutely essential. Excellent image quality can be achieved on almost any high field MR scanner if one invests some time to optimize protocols and image quality.
An external phased array surface coil providing simultaneous coverage of the abdomen and pelvic should be used to improve signal and image quality. Typically this requires a surface coil large enough to provide at least 50 cm in the cranio-caudal direction. Using the large body coil without a phased array surface coil is not an acceptable option.
MRI peritoneal protocol
General principles
Our protocol for peritoneal imaging is optimized for depicting small peritoneal tumors (21,27). All images are obtained during suspended respiration to minimize breathing artifact that can obscure subtle peritoneal tumors or inflammation. Faster pulse sequences that facilitate breath hold imaging also decrease the overall examination time which is essential when using intraluminal contrast material to distend to small bowel and colon. Other key elements that improve tumor depiction are fat suppression and high spatial resolution. Fat suppression is utilized for T2-weighted imaging, DWI, and all gadolinium-enhanced images. By suppressing the high signal intensity fat small peritoneal tumors and inflammation become more conspicuous.
An important TIP regarding image presentation on the PACS system
The axial images for the abdomen and pelvis are prescribed and acquired as separate acquisitions at the time of the scan. The MR technologist should combine the set of axial images from the abdomen with those from the pelvis so that the radiologist can scroll through one set of images that starts at the diaphragm and extends through the rectum for each image type. This will greatly simplify reading the examination. The MR technologist should ultimately send to the PACS one stack of axial images for T1, T2, diffusion, 3D fast spoiled gradient-echo (FSPGR) early gadolinium enhanced images, and 2D spoiled gradient-echo (SGE) delayed gadolinium enhanced images. Each stack includes the images of the abdomen and pelvis.
Table 1 lists the specific imaging parameters for our current peritoneal MRI protocol. In summary the examination includes axial dual echo T1 SGE images, fat suppressed T2-weighted Single shot FSE imaging, and breath hold DWI using an intermediate b-value of 500 s/mm2. Following injection of 10 mmol/kg intravenous gadolinium we obtain fat suppressed 3D FSPGR images in the axial plane twice thru the abdomen and pelvis. Coronal and sagittal 3D FSPGR imaging is performed. The final set of images is the axial 2D SGE with fat suppression. We find these images are less sensitive to breathing and motion artifact which is common at the end of the study. The 2D SGE images provide very sharp anatomic detail. The fat suppressed 2D SGE images are obtained about 5 minutes after the injection of gadolinium when slowly enhancing peritoneal tumors are most conspicuous.

Full table
Contrast mechanisms for peritoneal tumor depiction
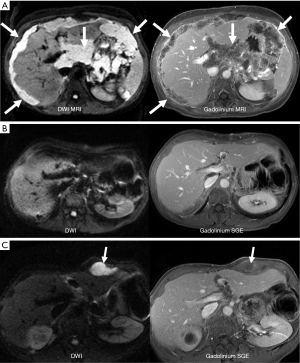
MR imaging is unique in its ability to show soft tissues using many different types of contrast. By modifying imaging parameters when setting up a scan one can accentuate tumors using T1-weighted, T2-weighted, or diffusion weighted contrast. One can also administer exogenous contrast intravenously to depict tumor enhancement. Each will produce an image that shows the tumor differently. In our experience DW imaging and gadolinium contrast-enhanced images are more useful for showing subtle peritoneal tumors (Figure 3).
Diffusion-weighted (DW) MR imaging
Diffusion is a physical property that describes the microscopic random movement of molecules in response to thermal energy (32). Also known as Brownian motion, diffusion may be affected by the biophysical properties of tissues such as cell organization and density, microstructure and microcirculation. DW imaging utilizes pulse sequences and techniques that are sensitive to very small-scale motion of water protons at the microscopic level. Single shot echo planar imaging (EPI) DW imaging is utilized to provide very rapid imaging sensitive to subtle small-scale alternations in diffusion. Areas of restricted water diffusion are displayed as areas of high signal intensity (41-49) (Figure 4).
Oncologic applications of DW imaging take advantage of restricted diffusion shown by most tumors (32,33). The higher cellularity of solid tumors and their increase in cell membranes per unit volume results in restriction of water movement and corresponding high signal intensity on DW images.
Abdominal DW imaging can be performed on commercially available high field MR systems. Most vendors currently utilize a single shot spin-echo EPI pulse sequence for DW imaging. DW imaging can be performed as a breath hold acquisition or as a breathing averaged acquisition with multiple excitations (32,33). The later may be acquired as a free breathing or respiratory triggered acquisition (32,33).
The sensitivity of the DW imaging sequence to water motion can be varied by changing the b-value which depends on the amplitude and the timing of the paired bipolar diffusion sensitizing gradients (37). One typically acquires at least two b-values of 0 s/mm2 combined with a second intermediate to a high b-value of 400 to 1,000 s/mm2. Acquiring additional b-values will improve the accuracy of the quantitative data obtained from DW imaging. Higher b-values result in more diffusion weighting with better background suppression, at the expense of reduced signal and increasing artifacts (32,33). At our institution we typically use b-value 0 s/mm2 combined with intermediate b-values of 500 s/mm2. For anatomic DW imaging these intermediate b-values achieve reasonable diffusion weighting while maintaining good image quality. Fat suppression is implemented to improve the contrast to background ratio on the DW images.
Tips for optimizing diffusion image quality
- Reduce the time of the read out acquisition by using the shortest echo time (TE) possible and by using parallel imaging techniques;
- Use spectral presaturation with inversion recovery (SPIR) fat suppression techniques which allows one to see the background anatomic detail. Inversion recovery (STIR) fat suppression will suppress background to the point that the anatomy is difficult to see;
- Use a high resolution to show small peritoneal tumors. We routinely use a matrix of 192×224. Notice that the phase is 224 and the frequency is 192 which also shortens the read out gradient time and reduces artifact;
- Use image inhomogeneity correction software to process the images making them more homogeneous. This processing software is available on the scanner and may be called phased array uniformity enhancement (PURE) or constant appearance level (CLEAR) on different vendors equipment;
- Use breath-hold DW imaging to reduce motion artifact;
- Optimize the DW scan so that you can image the abdomen in the axial plane in 1 breath hold and the pelvis in a second breath hold;
- Use diffusion direction selection that combines the X, Y, and Z diffusion gradient signal into a single orthogonal gradient. This will increase the signal and efficiency for breath-hold DW imaging. This DW parameter is called 3 in 1 or gradient overplus on different scanners.
Gadolinium-enhanced MR imaging
Peritoneal tumors enhance with intravenous gadolinium increasing their conspicuity so that very small tumor are depicted easily (28,29) (Figure 1). Peritoneal tumors enhance slowly so that they may not be visible on early arterial phase images but are best depicted on the final set of images obtained at about 5 minutes following gadolinium administration. For this reason the final set of axial 2D SGE is most important to achieve perfect breath-holding. If the patient is breathing small peritoneal tumors will be masked. These final set of images should be repeated if there is any motion artifact.
To depict small tumors a reasonably high in plane resolution must be balanced against the requirements for times short enough to allow for breath hold imaging. Our current post contrast imaging is performed with 3D FSGPR images obtained an in plane resolution of 320×256. The delayed axial 2D SGE images are obtained with an interpolated resolution of 512×256. In our experience peritoneal tumors often present as sheets for tumor cells lining the peritoneal surfaces rather than as solitary discrete small tumor nodules. In this setting high contrast resolution is probably more essential to distinguish thin sheets of tumor from normal anatomic structures. Increasing the in plane resolution, while maintaining the same breath hold time, can be achieved by using a higher bandwidth and or acceleration factor.
Tip for gadolinium-enhanced images: high resolution 320×256 detailed, breath-hold gadolinium-enhanced images are the goal for peritoneal imaging.
Optimal fat suppression on the gadolinium-enhanced images will also facilitate depiction of small peritoneal tumors by suppressing the adjacent high signal intensity of mesenteric, retroperitoneal, and abdominal wall fat. One may use chemical selective fat suppression. We currently use sequences that take implement a Dixon fat and water separation technique for more robust fat suppression. These 3D sequences are called Liver Acquisition with Volume Acceleration-eXtended Volume (LAVA FLEX) (General Electric Medical Systems), M-Dixon (Philips Medical), and T1 Dixon (Siemens Medical).
These images typically show more homogeneous fat suppression, sharper anatomic detail, less sensitivity of susceptibility artifact, and slightly better signal to noise ratio. Problems with fat and water swapping have been much improved on the most recent versions of the Dixon sequences (48).
MR image interpretation
Unenhanced T1 and T2-weighted images may show larger peritoneal tumor nodules and masses but are relatively insensitive for the depiction of small peritoneal tumors, carcinomatosis, and peritonitis (19). Following the intravenous injection of non specific extracellular gadolinium chelates peritoneal inflammation and peritoneal tumors enhance slowly. Peritoneal enhancement is thus best visualized on delayed images obtained 5 minutes after gadolinium injection. Normal peritoneal tissues are relatively thin measuring <3 mm in thickness and typically show only mild enhancement that is less than or equal to that of the liver parenchyma. Moderate to marked peritoneal enhancement and associated thickening is abnormal and is the hallmark of peritonitis or peritoneal carcinomatosis. It should be noted that the distinction between peritoneal inflammation and peritoneal tumor is based upon the clinical presentation since the MR imaging findings can be identical. Peritoneal thickening from tumors may be thin and regular, nodular, or mass-like. Peritonitis usually presents as smooth and regular peritoneal thickening and enhancement without dominant masses or nodules (29).
DW MR images are also very useful for depicting peritoneal diseases (34,35,41-47). Single shot EPI DW images using an intermediate b-value of 500 s/mm2 show restricted diffusion with peritoneal tumors and inflammation. On DW images ascites and bowel contents are suppressed while peritoneal and serosal tumors show restricted diffusion and are depicted as areas of high signal intensity. Suppression of ascites and bowel contents improves the conspicuity of peritoneal and serosal tumors on DW images. We have found that the most accurate examination for detecting peritoneal tumors is the combination of DW imaging and delayed gadolinium-enhanced MRI (18,19). The DW images are more easily interpreted when viewed in conjunction with the conventional MR images which provided better depiction of anatomic landmarks. Mesenteric tumors, bowel serosa tumors, and tumors involving the peritoneal reflections around the liver and pancreas were usually better seen on the DW images due to the high contrast of peritoneal tumors on these images. When comparing the b0 and b500 DW images one may see an interesting reversal of signal intensity. On the b0 images bowel contents are hyperintense while the bowel wall and serosal tumors are low signal intensity. On the corresponding b500 image the bowel contents are suppressed and the serosal and peritoneal tumor becomes hyperintense. The DW images are also useful to demonstrate associated lymphadenopathy, hepatic, and osseous metastases (32).
Preoperative evaluation of patients being considered for surgical cytoreduction and HIPEC
Preoperative MRI and CT of the abdomen and pelvis play an integral role in determining the extent of peritoneal and visceral disease in patients being considered for cytoreductive surgery (CRS) and HIPEC for appendiceal, ovarian, colorectal, primary peritoneal, gastric, mesothelioma and other rare types of gastrointestinal disease involving the peritoneum (18-29). Accurate preoperative imaging can assist in patient selection by avoiding surgery in patients whose tumors are too extensive for adequate surgical cytoreduction. Operative planning can also be optimized by assessing the volume and distribution of tumor depicted on preoperative imaging. In our experience accurate determination of the tumor involving the mesentery and bowel serosal is critical for both patient selection and preoperative planning. Accurate imaging prediction of the operative and pathologic PCI is the gold standard against which imaging studies are measured for patients undergoing surgical cytoreduction and HIPEC.
Although CT is routinely used at almost all medical centers for peritoneal cancer assessment, its shortcomings in accurately detecting peritoneal tumors are well documented (Figures 1 and 3). Chua et al. (50) found that the accuracy in depicting peritoneal lesions using CT regardless of size, ranged from 51% to 88% in the 9 abdominopelvic regions and 21-25% in the four small intestinal regions in pseudomyxoma peritonei. In comparing the radiologic CT PCI to the operative PCI in the study, the radiologic PCI consistently under estimated the volume of peritoneal disease. Koh et al. (37) in another study reported that CT identified the presence of disease and portrayed true lesion size in only 60% of the cases of colorectal carcinoma. Small nodules, <0.5 cm were visualized on CT with only a sensitivity of 11%. Radiologic PCI scores significantly underestimated the intraoperative PCI with the operative score almost double the radiologic PCI score. CT detection of peritoneal nodules was 67% in the epigastrium, 54% in the right upper quadrant and 60% in the pelvis. Small bowel involvement had the least sensitivity of all the regions 8-17%. A recent review of multidetector CT for preoperative determination of PCI in patients with primary and recurrent ovarian cancer found a regional sensitivity of 66% and accuracy of 77% for peritoneal tumor compared to histological findings (24). On a patient level analysis the sensitivity for small bowel/mesenteric tumor (regions 9-12) was 58%.
MRI provides a much more accurate imaging examination for preoperative evaluation of patients being assessed for cytoreduction and HIPEC. Previously we have compared the preoperative MRI PCI and surgical PCI in 33 patients and found no significant difference (18). MRI correctly categorized the tumor volume found at surgery in 29 (88%) if 33 patients. MRI accurately categorized the tumor volume as small volume (PCI 0-9) in 89% of patients, moderate volume (PCI 10-20) in 75% of patients, and large volume (PCI >20) in 90% of patients. Klumpp et al. (22) found that the results of preoperative MRI correlated well with the surgical PCI, postoperative resection status, and survival time. They suggested that MRI might be a suitable patient’s selection tool as patient outcome correlated with the extent of peritoneal carcinomatosis found on the preoperative MRI.
In a retrospective comparison of CT and MRI compared to the surgical PCI we found that the CT PCI underestimated the surgical PCI in 19 of 22 patients (19). The median surgical PCI was 33 compared to median CT PCI of 15. The median percentage difference between the surgical PCI and the CT PCI was 50% compared to 6% for the MRI PCI versus the surgical PCI. Compared to the surgical PCI, MRI PCI correctly categorized tumor volume in 91% of the patients as opposed to only 50% with CT scanning. Notably in the small bowel areas (sites 9-12) MRI had an accuracy of 92% versus 48% for CT. Overall, surgery confirmed 222 sites of tumor. MRI demonstrated per site sensitivity of 0.95, specificity 0.70 and accuracy 0.88. CT showed a corresponding per site sensitivity 0.55, specificity 0.86, and accuracy 0.63 (19).
Surveillance MR imaging following CRS and HIPEC
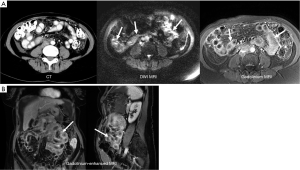
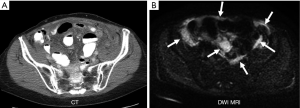
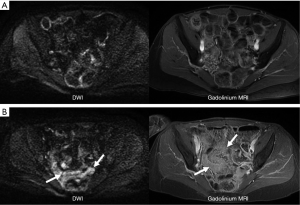
Despite successful treatment local intraperitoneal recurrence of tumor occurs in 28-44% of patients with appendiceal cancer and remains a significant problem that reduces overall survival (51-53) (Figure 5). Recurrence rates can be much higher for other tumor histology, higher grade tumors, or patients with higher initial PCI scores. Second and third complete cytoreduction with repeated HIPEC for patients with recurrent tumor has been advocated as the best approach to achieve improved overall survival (51). The early detection of recurrent tumor on serial laboratory tests and imaging studies plays a critical role in identifying patients who should be considered for repeat CRS and HIPEC (51-53).
Assessment of response following CRS and HIPEC using serial tumor markers alone is challenging (54,55). In one study preoperative CEA and CA 19.9 levels were increased in 75% and 58% of patient’s pseudomyxoma peritonei (53) and during follow up a high CA 19.9 level was more predictive of recurrence. However, a progressive rise in serum tumor markers with disease recurrence is reliably seen in only some patients and does not predict the volume of tumor recurrence or its location. Some patients with large volume recurrent tumor may not show an elevation in tumor markers. Tumor markers do not reliably monitor disease stabilization, partial response, or continued complete response (56).
In a longitudinal study of 50 patients with appendiceal neoplasm (DPAM 13, PMCA 37) MR imaging detected tumor recurrence earlier than serial tumor markers (27). Following CRS and HIPEC patients entered follow-up surveillance with serial MRI every 6 months and serial laboratory studies including CA 125, CEA, and CA19-9. During surveillance tumor recurrence was documented in 30 (0.60) patients with median time to recurrence of 13 months (range, 5-56 months). MRI detected recurrent tumor in 28 patients including 11 patients with normal laboratory values (sensitivity 0.93, specificity 0.95, accuracy 0.94, PPV 0.97, and NPV 0.90). Serial laboratory values showed tumor recurrence in 14 patients (sensitivity 0.48, specificity 1.00, accuracy 0.69, PPV 1.0, and NPV 0.57). Median survival was 50 months for 11 patients with earlier MRI detection of recurrence vs. 33 months for the other 19 patients with recurrence (27).
Earlier intervention performed with smaller volume tumor should yield better surgical results with lower morbidity and mortality (16). If one were to wait until the tumor burden is large enough to be detected on a CT scan, the delay in treatment could adversely affect outcome and survival. The importance of repeat CRS and HIPEC for treating recurrent appendiceal cancer has been described in prior reports (51,52). Complete cytoreduction after repeat surgery was the only independent prognostic factor for improved survival resulting in a 70% 5-year survival rate for 402 patients with appendiceal cancer (51).
At our institution we routinely follow patients with serial serum laboratory values and a surveillance MRI every 6 months (Figure 6).
Interpretation of surveillance MR examinations
Following CRS and HIPEC some peritoneal and bowel serosal thickening is present with enhancement on gadolinium-enhanced MRI and DW imaging. These changes reflect normal post operative findings. Therefore, the initial MR examination following CRS and HIPEC establishes the patient post operative baseline. Assuming that the surgeon achieved an R0 tumor resection, at this initial surveillance examination all findings represent normal anatomy and post surgical changes.
Tip: use the initial post HIPEC MRI to establish the patients post treatment baseline. Look for changes from this normal baseline on follow up surveillance MR examinations.
On subsequent MR examinations we carefully assess for any interval changes that indicate disease progression or recurrence. These concerning findings include obvious tumor masses, but more commonly one sees increasing peritoneal thickening, peritoneal nodules, and ascites. Identifying tumor on both gadolinium-enhanced MR images and DW images improves our confidence and accuracy in detecting recurrent tumor.
An important point is that MR findings attributable to post surgical changes do not progress on serial MR examinations. If one observes progressively worsening bowel wall thickening and mesenteric infiltration on gadolinium-enhanced MRI and DW images this represents tumor recurrence. Post surgical changes will gradually resolve on serial MR exams and will not show progression. This assessment assumes that the patient is clinically stable and that there are not superimposed acute diseases such as a gastroenteritis or post operative abscess.
Tip: post operative changes are common following cytoreduction and HIPEC but should be stable or more commonly resolve on subsequent surveillance MR examinations. Progressive changes indicate tumor recurrence (Figure 6).
Limitations of peritoneal MRI
Despite the clear superiority of MRI over CT the major detractors against MRI are the longer exam times and the cost of the MRI. It takes on the average 30 minutes to perform the procedure and which can be influenced by motion artifacts related to respiration and bowel peristalsis. We routinely use Glucagon or levsin to decrease peristalsis and encourage breath holding. The charges for the MRI in our institution are considerably less than that or a CT scan.
MRI of peritoneal tumor is less familiar to surgeons and requires some training for the radiologist to interpret accurately and with confidence (26). Less experienced radiologists will likely prefer CT for its simplicity and ease of interpretation. A trained abdominal MR radiologist will use the superior contrast resolution of MR to routinely detect subtle peritoneal tumors allowing for more accurate preoperative evaluation. Ongoing collaboration between the surgical oncologist and the MRI radiologist will develop a relationship that will facilitate communication and education and will ultimately improve patient care.
Conclusions
With MR imaging of peritoneal tumor, the devil is truly in the details. Performing and interpreting the examination correctly requires careful attention to detail. A trained MR staff and experienced MR radiologist are key elements in this team effort. The end result is an MR examination that more accurately determines the extent of the peritoneal tumor. In our experience incorporation of MRI into our peritoneal patient evaluations results in improved patient selection prior to CRS and HIPEC and earlier detection of recurrence during surveillance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am 2003;12:703-27. xiii. [PubMed]
- Vaira M, Cioppa T, DE Marco G, et al. Management of pseudomyxoma peritonei by cytoreduction+HIPEC (hyperthermic intraperitoneal chemotherapy): results analysis of a twelve-year experience. In Vivo 2009;23:639-44.
- Votanopoulos KI, Russell G, Randle RW, et al. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol 2015;22:1274-9. [PubMed]
- Winder T, Lenz HJ. Mucinous adenocarcinomas with intra-abdominal dissemination: a review of current therapy. Oncologist 2010;15:836-44. [PubMed]
- Graziosi L, Marino E, Donini A. Role of CRS plus HIPEC in gastric cancer peritoneal carcinomatosis. J Surg Oncol 2015;111:248. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [PubMed]
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [PubMed]
- Alexander HR Jr, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779-86. [PubMed]
- Turner K, Varghese S, Alexander HR Jr. Current concepts in the evaluation and treatment of patients with diffuse malignant peritoneal mesothelioma. Current concepts in the evaluation and treatment of patients with diffuse malignant peritoneal mesothelioma. J Natl Compr Canc Netw 2012;10:49-57. [PubMed]
- Chua TC, Yan TD, Deraco M, et al. Multi-institutional experience of diffuse intra-abdominal multicystic peritoneal mesothelioma. Br J Surg 2011;98:60-4. [PubMed]
- Sugarbaker PH. Five reasons why cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy must be regarded as the new standard of care for diffuse malignant peritoneal mesotheliomia [corrected]. Ann Surg Oncol 2010;17:1710-2; author reply 1713-4.
- Helm CW, Richard SD, Pan J, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer: first report of the HYPER-O registry. Int J Gynecol Cancer 2010;20:61-9. [PubMed]
- Glehen O, Gilly FN. Quantitative prognostic indicators of peritoneal surface malignancy: carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg Oncol Clin N Am 2003;12:649-71. [PubMed]
- Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2005;2:3. [PubMed]
- Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995;221:124-32. [PubMed]
- Yan TD, Sim J, Morris DL. Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2007;14:1807-17. [PubMed]
- Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2012;19:1394-401. [PubMed]
- Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2015;22:1708-15. [PubMed]
- Ricke J, Sehouli J, Hach C, et al. Prospective evaluation of contrast-enhanced MRI in the depiction of peritoneal spread in primary or recurrent ovarian cancer. Eur Radiol 2003;13:943-9. [PubMed]
- Low RN, Barone RM, Gurney JM, et al. Mucinous appendiceal neoplasms: preoperative MR staging and classification compared with surgical and histopathologic findings. AJR Am J Roentgenol 2008;190:656-65. [PubMed]
- Klumpp B, Aschoff P, Schwenzer N, et al. Correlation of preoperative magnetic resonance imaging of peritoneal carcinomatosis and clinical outcome after peritonectomy and HIPEC after 3 years of follow-up: preliminary results. Cancer Imaging 2013;13:540-7. [PubMed]
- Klumpp BD, Aschoff P, Schwenzer N, et al. Peritoneal carcinomatosis: comparison of dynamic contrast-enhanced magnetic resonance imaging with surgical and histopathologic findings. Abdom Imaging 2012;37:834-42. [PubMed]
- Mazzei MA, Khader L, Cirigliano A, et al. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom Imaging 2013;38:1422-30. [PubMed]
- Klumpp BD, Schwenzer N, Aschoff P, et al. Preoperative assessment of peritoneal carcinomatosis: intraindividual comparison of 18F-FDG PET/CT and MRI. Abdom Imaging 2013;38:64-71. [PubMed]
- Torkzad MR, Casta N, Bergman A, et al. Comparison between MRI and CT in prediction of peritoneal carcinomatosis index (PCI) in patients undergoing cytoreductive surgery in relation to the experience of the radiologist. J Surg Oncol 2015;111:746-51. [PubMed]
- Low RN, Barone RM, Lee MJ. Surveillance MR imaging is superior to serum tumor markers for detecting early tumor recurrence in patients with appendiceal cancer treated with surgical cytoreduction and HIPEC. Ann Surg Oncol 2013;20:1074-81. [PubMed]
- Low RN, Duggan B, Barone RM, et al. Treated ovarian cancer: MR imaging, laparotomy reassessment, and serum CA-125 values compared with clinical outcome at 1 year. Radiology 2005;235:918-26. [PubMed]
- Elsayes KM, Staveteig PT, Narra VR, et al. MRI of the peritoneum: spectrum of abnormalities. AJR Am J Roentgenol 2006;186:1368-79. [PubMed]
- Coakley FV, Choi PH, Gougoutas CA, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology 2002;223:495-9. [PubMed]
- Low RN, Barone RM, Lacey C, et al. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology 1997;204:513-20. [PubMed]
- Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007;188:1622-35. [PubMed]
- Malayeri AA, El Khouli RH, Zaheer A, et al. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics 2011;31:1773-91. [PubMed]
- Low RN, Gurney J. Diffusion-weighted MRI (DWI) in the oncology patient: value of breathhold DWI compared to unenhanced and gadolinium-enhanced MRI. J Magn Reson Imaging 2007;25:848-58. [PubMed]
- Low RN, Sebrechts CP, Barone RM, et al. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings--a feasibility study. AJR Am J Roentgenol 2009;193:461-70. [PubMed]
- Esquivel J, Chua TC, Stojadinovic A, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol 2010;102:565-70. [PubMed]
- Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009;16:327-33. [PubMed]
- Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 2004;363:345-51. [PubMed]
- Brenner D, Elliston C, Hall E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 2001;176:289-96. [PubMed]
- Pfannenberg C, Königsrainer I, Aschoff P, et al. (18)F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2009;16:1295-303. [PubMed]
- Kyriazi S, Collins DJ, Messiou C, et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging--value of histogram analysis of apparent diffusion coefficients. Radiology. 2011;261:182-92. [PubMed]
- Kyriazi S, Collins DJ, Morgan VA, et al. Diffusion-weighted imaging of peritoneal disease for noninvasive staging of advanced ovarian cancer. Radiographics 2010;30:1269-85. [PubMed]
- Bonekamp S, Corona-Villalobos CP, Kamel IR. Oncologic applications of diffusion-weighted MRI in the body. J Magn Reson Imaging 2012;35:257-79. [PubMed]
- Bozkurt M, Doganay S, Kantarci M, et al. Comparison of peritoneal tumor imaging using conventional MR imaging and diffusion-weighted MR imaging with different b values. Eur J Radiol 2011;80:224-8. [PubMed]
- Fischerova D, Burgetova A. Imaging techniques for the evaluation of ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2014;28:697-720. [PubMed]
- Hanbridge A, Mester U. Mesentery, Omentum, Peritoneum: CT, ultrasound, and MRI. In: Hamm B, Ross PR, editors. Abdominal Imaging. Berlin: Springer-Verlag Berlin Heidelberg, 2013:1535-40.
- Hameeduddin A, Sahdev A. Diffusion-weighted imaging and dynamic contrast-enhanced MRI in assessing response and recurrent disease in gynaecological malignancies. Cancer Imaging 2015;15:3. [PubMed]
- Low RN, Panchal N, Vu AT, et al. Three-dimensional fast spoiled gradient-echo dual echo (3D-FSPGR-DE) with water reconstruction: preliminary experience with a novel pulse sequence for gadolinium-enhanced abdominal MR imaging. J Magn Reson Imaging 2008;28:946-56. [PubMed]
- Espada M, Garcia-Flores JR, Jimenez M, et al. Diffusion-weighted magnetic resonance imaging evaluation of intra-abdominal sites of implants to predict likelihood of suboptimal cytoreductive surgery in patients with ovarian carcinoma. Eur Radiol 2013;23:2636-42. [PubMed]
- Chua TC, Al-Zahrani A, Saxena A, et al. Determining the association between preoperative computed tomography findings and postoperative outcomes after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 2011;18:1582-9. [PubMed]
- Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol 2007;14:2289-99. [PubMed]
- Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol 2008;98:295-9. [PubMed]
- Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg 2007;245:104-9. [PubMed]
- van Ruth S, Hart AA, Bonfrer JM, et al. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2002;9:961-7. [PubMed]
- Ross A, Sardi A, Nieroda C, et al. Clinical utility of elevated tumor markers in patients with disseminated appendiceal malignancies treated by cytoreductive surgery and HIPEC. Eur J Surg Oncol 2010;36:772-6. [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [PubMed]


