A combined modality therapeutic approach to metastatic anal squamous cell carcinoma with systemic chemotherapy and local therapy to sites of disease: case report and review of literature
Introduction
Anal carcinoma is a relatively rare malignancy and metastatic presentation accounts for only 10–20% of patients (1). Patients presenting with metastatic anal cancer typically receive palliative treatment with systemic chemotherapy; however, control of the primary tumor is important as local failure patterns may result in significant morbidity. A combined strategy of systemic and local therapies may prove beneficial in selected cases. In metastatic colorectal cancer management resection of isolated liver metastases is increasingly utilized as an effective treatment strategy in select patients; however, the utilization in patients with metastatic anal cancer is not as developed (2). This paper reviews a case of metastatic anal squamous cell cancer with liver metastases treated with systemic chemotherapy followed by hepatic resection of an isolated metastasis and subsequent concurrent chemoradiotherapy to the locoregional disease after achieving a good response to systemic therapy. Unique to this case is the initial presentation with significant invasion into the prostate and base of penis, with associated symptoms. We also present a review of existing literature regarding the combination of systemic and localized treatment of metastatic anal cancer.
Case presentation
A 55-year-old Caucasian male with a history of controlled hypertension, and previous tobacco use presented with atypical chest pain for one day duration. He reported a one year history of pencil thin stools, bright red blood per rectum, and a feeling of rectal fullness which he attributed to hemorrhoids and did not seek medical attention. He also reported urinary frequency, erectile dysfunction, and a 10-pound weight loss in the month prior to presentation. On physical exam he had no abdominal masses or tenderness but his rectal exam revealed a tender firm nodular mass at the anal verge advancing along the left side of the rectum. Cardiac work up for his atypical chest pain was normal but a computed tomography (CT) incidentally detected a 2.5-cm lesion in the right hepatic lobe. Magnetic resonance imaging (MRI) of the abdomen with contrast showed a region in the right hepatic lobe with delayed enhancement, increased T2 signal intensity, diffusion signal abnormality, and associated capsular retraction.
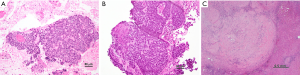
A CT guided biopsy of the liver lesion was performed, and pathology returned positive for metastatic carcinoma (Figure 1) with stains positive for p63, cytokeratin (CK) 5/6 and p16 suggesting squamous cell histology (3). p16 expression status determined by immunohistochemical staining is a useful surrogate biomarker for HPV integration (4). Endoscopic evaluation revealed a 7-cm anorectal mass and biopsy was consistent with moderately differentiated invasive squamous cell carcinoma, histologically similar to the liver lesion (Figure 1A,B). Histologic features seen such as poor keratinization, prominent basaloid features (small to moderate amounts of cytoplasm, peripheral palisading, retraction artifact, and eosinophilic necrosis in center of nests) and small tumor cell size, are consistent with HPV infection (3,5). This combined with the diffuse p16 suggest an association with high risk HPV infection. HPV infection is associated with 80–85% of anal cancer and diagnoses with HPV 16 being the most common subtype detected (approximately 70% of cases). HPV positive anal tumors tend to be more responsive to chemoradiotherapy (1). Testing for the human immunodeficiency virus (HIV) was negative in our patient.
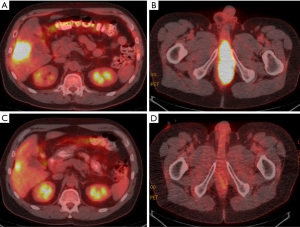
A positron emission tomography (PET) CT study showed the hyper metabolic liver lesion with a maximum standard uptake value (max SUV) of 14.6 (Figure 2A) and a large 12×5 cm soft tissue mass originating from the recto prostatic pouch with significant hyper metabolic activity with a max SUV of 25 (Figure 2B). The mass had significant local invasion and extended through the inferior prostate involving the base of the corpus spongiosum. The final American Joint Commission on Cancer staging was T4N0M1, stage IV.
The case was reviewed by a multidisciplinary tumor board. Given his age, lack of significant comorbidities, good performance status (Eastern Cooperative Oncology Group 1) and limited metastatic disease, recommendations were to proceed with systemic chemotherapy followed by re-evaluation for consideration of resection of the hepatic lesion and definitive treatment of his locoregional disease if a good clinical response was noted.
He received cisplatin 100 mg/m2 IV (day 2) and 5-fluorouracil (FU) continuous infusion 1,000 mg/m2/days 1–5 (over 96 hours) every 4 weeks for two cycles. Restaging scans were obtained at the completion of two months of systemic therapy. The images demonstrated a significant reduction in the size of the pelvic mass (now 3.5×11.0 cm, previously 5.1×12.0 cm) and in hyper metabolic activity (max SUV of 5.6, previously max SUV 25). Although the liver metastasis was unchanged in size at 2.6 cm, there was a significant reduction in hyper metabolic activity (max SUV 5.5, previously 14.6) as demonstrated by Figure 2C suggesting treatment response. The MRI pelvis showed a residual 6×2.2 cm anal mass extending into the base of penis and base of prostate with evidence of interval fibrosis and scarring, consistent with treatment response. Improvement of urinary symptoms was also noted. Given the treatment response, symptom improvement and absence of new metastases, the tumor board recommended resection of the liver lesion followed by definitive concurrent chemoradiotherapy to the anal mass with the goal of long term disease control.
The patient underwent laparoscopic partial right lobectomy 34 days after his last dose of chemotherapy. Pathology of the partially resected right lobe showed extensive foreign body giant cell reaction, fibrosis, necrosis, and no evidence of residual carcinoma, consistent with pathologic complete response (Figure 1C). Bladder cystoscopy was performed demonstrating a 4-cm stricture associated with tumor involvement of the prostate and base of penis. Because of concern for potential urinary obstruction or complications, a suprapubic catheter was placed for urinary diversion at the time of surgery.
One month following surgery, he began definitive concurrent chemoradiotherapy to the primary tumor and regional lymph nodes. Intensity modulated radiation therapy plan consisted of 30 fractions of 1.8 gray (Gy) per fraction to the primary disease and 1.5 Gy to uninvolved lymph nodes for a total dose of 54 and 45 Gy respectively. As there were no involved lymph nodes, no interval 50.4 Gy dose was administered. The high dose (54 Gy) radiotherapy volume encompassed all areas of disease present prior to initiating induction chemotherapy, extending 2 cm proximal to the areas of uptake on PET scan and MRI, and involving approximately 1/3 of the proximal penis and the entire prostate including the proximal urethra. Concurrent chemotherapy was given with continuous 5-FU 1,000 mg/m2/day (days 1–4 and 29–32) and mitomycin C 10 mg/m2 (day 1, 29), without significant toxicity. Post chemoradiotherapy PET CT imaging showed reduction in size and hypermetabolic activity of the local disease (Figure 2D). Although the patient was able to urinate without significant difficulty throughout most of the initial course of radiotherapy, the suprapubic catheter was increasingly needed toward the end of radiotherapy.
Surveillance PET scan and MRI were performed at 3 month follow up demonstrating a good response to treatment with a maximum SUV of 3 in the area of the anal canal, reduction in the size of the mass invading the prostate to 1.1 cm as compared with 2.1 cm prior to initiating concurrent chemo radiotherapy. Interval follow-up scans, physical examination, and endoscopic ultrasounds with biopsy through 19 months of follow-up have demonstrated no evidence of persistent or recurrent disease. Urinary trials were successfully initiated, and the suprapubic catheter was removed 4 months after completion of radiotherapy, with self-catheterization procedures tolerated as needed.
Discussion
Anal cancer is an uncommon malignancy with a reported incidence of 7,000 new cases in the US in 2014, and accounts for 2% of all gastrointestinal malignancies (6). Although this has historically affected more women than men, recent data suggests increasing incidence in both genders with a more pronounced increase in men. A higher incidence rate (2.71/100,000) and lower survival have been reported for African American men. Patients with metastatic disease have a poor five year overall survival (18%) compared to patients with localized disease (78%) (7). Median survival for metastatic anal carcinoma is approximately 12 months, however this novel treatment approach has resulted in a longer than average progression free interval of 19 months for our patient (8-11).
Initial presentation with distant metastatic disease accounts for 5–8% of patients diagnosed with anal cancer and metastatic progression following primary treatment is seen in about 10–20% of cases. Common sites of extra pelvic metastasis are the liver, lung, and extra pelvic lymph nodes (1). The liver is the most common metastatic site (11). Treatment of patients with oligometastatic disease should be individualized and discussed in a multidisciplinary setting.
Though mitomycin C and 5FU are evidence based regimens for definitive treatment using concurrent chemoradiotherapy, optimal chemotherapy regimen for treatment of metastatic anal cancer is not well defined. A commonly used regimen is 5FU given as a continuous infusion over 5 days at a dose of 1,000 mg/m2/day with 100 mg/m2 of cisplatin on day 2 and cycles repeated every 4 weeks (9). Several other regimens have been reported in the treatment of anal carcinoma as well, with comparisons to cisplatin and 5FU combination. In a case series reported by Tanum the cisplatin and 5FU regimen was reported to have median survival of 12 months (range, 4–68 months), whereas an alternative regimen, of mitomycin C and 5FU had a median overall survival of 11 months (range, 3–21 months). The difference reported was not statistically significant (11). In another retrospective review, Eng et al. reported that the cisplatin and 5FU regimens had better median OS and response rate than a regimen of carboplatin with paclitaxel, but the benefit was not statistically significant, and the regimens were not compared in a randomized controlled study design (12). The international multicenter phase II study in advanced anal cancer (interAACT study) is currently comparing cisplatin and 5 FU to a carboplatin and paclitaxel regimen for patients with metastatic anal cancer in a randomized study design. We utilized the induction regimen of cisplatin and 5FU with good radiographic (Figure 2) and pathologic results.
The combined modality approach of treating metastatic anal carcinoma with local modalities in addition to systemic chemotherapy, as used in our patient, has been reported to be associated with improved progression free survival. In 1999 Faivre et al. reported on 19 patients with metastatic anal cancer who were treated with continuous 5FU (1 g/m2/d for 5 days) and cisplatin (100 mg/m2 on day 2 every 4 weeks). A total of 10 patients had received further local therapies. Out of the 10 patients they reported that three patients had significantly benefited from the addition of local therapy. These patients were still alive at 4, 5, and 7 years at the time of reporting. This was a significant improvement from the median survival of 34.5 months reported from the study (9). Of the three patients one patient underwent hepatic resection followed by systemic chemotherapy, and the other two patients had initial systemic chemotherapy followed by surgery or radiation to the limited sites of disease. Other cases reported in the literature have shown similar benefits with the combined treatment approach resulting in improved progression free survival as summarized in Table 1 (9,11-15).

Full table
The ideal sequencing of chemotherapy, surgery and chemoradiation is not known, and various authors have taken different approaches. For example Lupinacci et al. treated their patient initially with induction chemotherapy followed by chemoradiation to the primary and concluded with resection of residual liver lesions (14). This approach is slightly different to our strategy where our patient was treated with induction chemotherapy followed by liver resection and later definitive local therapy to the primary tumor. The rationale for this sequence in our patient was to ensure control of the metastatic site through the liver resection, prior to pursuing aggressive local control of the primary disease with chemoradiotherapy. This is similar to the strategy reported by Mentha et al. with respect to metastatic rectal cancers with synchronous liver lesions (2). The optimal sequencing of chemotherapy, chemoradiotherapy and resection of metastatic disease is yet to be defined.
An important consideration is which patients are likely to benefit from a more aggressive treatment strategy that addresses both local disease and isolated metastases. The patient selection may be informed by the data reported by Pawlik et al. in their retrospective analysis on liver directed therapies for 27 patients with metastatic anal squamous cell carcinoma and isolated hepatic metastases. The majority of patients underwent hepatic resection (n=47), some radiofrequency ablation (n=3), and some received both radiofrequency ablation and surgery (n=2). The primary tumors were treated with chemoradiotherapy in the majority of cases (70%). Factors associated with poor disease free survival and disease specific death were reported as hepatic metastases >5 cm and a positive surgical margin. Synchronous presentation of metastases was associated with a fourfold increase in risk of disease specific death. Acknowledging the study was limited by size the authors concluded that patients with metachronous metastasis amenable to a margin negative resection may benefit from this combined therapeutic approach and have longer survival rates (16). However our patient had a synchronous metastatic presentation with a 3.3-cm liver lesion and has had a significant disease free interval. Optimal criteria for patient selection are yet to be well defined.
One novel aspect of our case is the presence of metastatic disease as well as locally advanced disease extending into the prostate and base of penis. There are no clear guidelines on what extent of at-risk tissue should be included in the radiotherapy plan. The decision to include 2 cm distal to the pre-treatment extent of PET positive tumor was based on (I) the involvement of the penis and corpus with no clear fascial barrier to prevent spread further into the corpus; and (II) the need to reduce risk of long term toxicity such as penile necrosis (17). A urethral stricture was noted prior to starting concurrent chemoradiotherapy and placement of a suprapubic catheter assisted toward the end of radiotherapy and in the initial follow-up months. In the year following completion of treatment, the patient has been able to tolerate removal of the suprapubic catheter and void with assistance using self-catheterization techniques. Although these complications may represent a chronic morbid condition, medical management has been well tolerated, and the overall control of local disease has been achieved.
Conclusions
The treatment strategy for metastatic anal carcinoma should be individualized. Here, we present a case of a highly locally invasive anal cancer with isolated metastases that responded well to systemic therapy followed by metastasectomy and definitive locoregional therapy. Key to management was a thorough review and discussion with a multidisciplinary team to assess the feasibility of local therapies to the metastatic and primary site. Based on this and other published reports it is clear that some patients benefit from the combined approach (Table 1). The ideal method of selecting such patients, however, is yet to be determined. The studies reporting on this approach are limited by their non-randomized, retrospective nature; with small sample sizes. Further studies investigating this approach are necessary, but plagued by feasibility issues, given the rarity of the presentation. However, this review may help refine optimal therapies for select patients with isolated metastases who have a good response to systemic therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:1165-76. [Crossref] [PubMed]
- Mentha G, Majno PE, Andres A, et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg 2006;93:872-8. [Crossref] [PubMed]
- Longacre TA, Rouse RV. “Squamous Cell Carcinoma of the Anus.” Differential Diagnosis. Stanford School of Medicine, 2015. Available online: http://surgpathcriteria.stanford.edu/gitumors/anus-dysplasia/printable.html
- Yhim HY, Lee NR, Song EK, et al. The prognostic significance of tumor human papillomavirus status for patients with anal squamous cell carcinoma treated with combined chemoradiotherapy. Int J Cancer 2011;129:1752-60. [Crossref] [PubMed]
- Shia J. An update on tumors of the anal canal. Arch Pathol Lab Med 2010;134:1601-11. [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: The Surveilance, epidemiology, and end results experience, 1973-2000. Cancer 2004;101:281-8. [Crossref] [PubMed]
- Ajani JA, Carrasco CH, Jackson DE, et al. Combination of cisplatin plus fluoropyrimidine chemotherapy effective against liver metastases from carcinoma of the anal canal. Am J Med 1989;87:221-4. [Crossref] [PubMed]
- Faivre C, Rougier P, Ducreux M, et al. 5-fluorouracile and cisplatinum combination chemotherapy for metastatic squamous-cell anal cancer. Bull Cancer 1999;86:861-5. [PubMed]
- Ghosn M, Kourie HR, Abdayem P, et al. Anal cancer treatment: current status and future perspectives. World J Gastroenterol 2015;21:2294-302. [Crossref] [PubMed]
- Tanum G. Treatment of relapsing anal carcinoma. Acta Oncol 1993;32:33-5. [Crossref] [PubMed]
- Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget 2014;5:11133-42. [Crossref] [PubMed]
- Gurfinkel R, Walfisch S. Combined treatment of basaloid anal carcinoma using cisplatin, 5-fluorouracil and resection of hepatic metastasis. Tech Coloproctol 2005;9:235-6. [Crossref] [PubMed]
- Lupinacci RM, Simon JM, Spano JP, et al. Aggressive strategy for the treatment of synchronous metastatic anal squamous cell carcinoma. Clin Res Hepatol Gastroenterol 2013;37:e127-9. [Crossref] [PubMed]
- Tokar M, Bobilev D, Zalmanov S, et al. Combined multimodal approach to the treatment of metastatic anal carcinoma: report of a case and review of the literature. Onkologie 2006;29:30-2. [Crossref] [PubMed]
- Pawlik TM, Gleisner AL, Bauer TW, et al. Liver-directed surgery for metastatic squamous cell carcinoma to the liver: results of a multi-center analysis. Ann Surg Oncol 2007;14:2807-16. [Crossref] [PubMed]
- Cicchini C, Stazi A, Ciardi A, et al. An unusual late radiotherapy-related complication requiring surgery in anal canal carcinoma. J Surg Oncol 2000;74:167-70. [Crossref] [PubMed]


