Deteriorated portal flow may cause liver failure in patients with hepatocellular carcinoma being treated with sorafenib
Introduction
Based on two phase III randomized trials showing that sorafenib, a multi-kinase inhibitor, prolonged overall survival in patients with advanced hepatocellular carcinoma (HCC) and Child-Pugh A cirrhosis, sorafenib was approved for the treatment of patients with advanced HCC (1,2). Although none of the patients in those studies developed liver failure during sorafenib treatment, several subsequent studies showed that sorafenib could cause liver failure, especially in patients with poorer Child-Pugh stages (3,4). To date, however, the clinical characteristics and subsequent clinical course of patients who experienced liver failure during treatment with sorafenib have not yet been clarified.
We recently encountered two patients with HCC who showed rapid progression of liver failure during sorafenib treatment. One had portal vein tumor thrombus (PVTT) and the other developed portal vein thrombosis (PVT) during the treatment. Although both PVTT and PVT are regarded as negative prognostic factors for patients with HCC, previous studies have identified several patients with deteriorated portal flow who achieved complete remission (CR) while being treated with sorafenib (5-17). We hypothesized that the pathogenesis of disease may be similar in HCC patients who achieve CR and those who experience liver failure while on sorafenib. A detailed information understanding of their clinical characteristics may contribute to a better understanding of the effects of sorafenib.
Case presentation
Patient 1
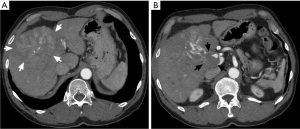
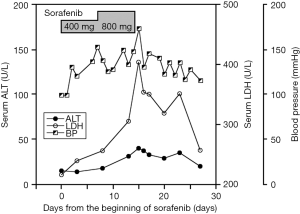
A 64-year-old man was admitted to our hospital for general fatigue. He had a more than 10-year history of alcohol abuse and had been diagnosed with alcoholic liver cirrhosis 4 years prior to admission. Contrast-enhanced computed tomography (CT) showed a 10 cm mass with high-low pattern in the right lobe of his liver and a tumor thrombus in the portal right branch extending to the main trunk, but no ascites (Figure 1). Based on imaging results, the patient was diagnosed with HCC. At admission, he had a total bilirubin concentration of 1.3 mg/mL, an alanine aminotransferase (ALT) concentration of 50 IU/L, a lactate dehydrogenase (LDH) concentration of 200 IU/L, an ammonia concentration of 67 µg/dL, and a prothrombin time international normalized ratio (PT-INR) of 1.10. His liver function was graded as Child-Pugh A. Serum concentrations of HCC tumor markers were markedly elevated, with a des-gamma-carboxy prothrombin concentration (DCP) of 1,171 mAU/mL and an α-fetoprotein concentration of 9,914 ng/mL. Initial treatment consisted of hepatic arterial infusion chemotherapy with 5-fluorouracil and cisplatin for 3 weeks, which resulted in stable disease. Following an interval of 3 weeks, the patient was started on sorafenib 400 mg/day for 7 days. Because the only adverse effect observed was a slight elevation in blood pressure (BP), his sorafenib dose was increased up to 800 mg/day. On the morning of day 14 of sorafenib treatment, the patient abruptly became disoriented, and his systolic BP increased to 180 mmHg (Figure 2). He experienced asterixis and his serum ammonia concentration increased to 153 µg/dL, suggesting that his disorientation was due to hepatic encephalopathy. Blood tests showed that his LDH had increased to 403 IU/L whereas his ALT remained 40 IU/L. Treatment with sorafenib was stopped on that day. The next day, his encephalopathy had diminished, with a normal ammonia level and BP, while his LDH concentration had decreased to 320 IU/L. The patient has since been treated for 4 months with the same regimen (5-fluorouracil and cisplatin) of hepatic arterial infusion chemotherapy without any further sign of hepatic encephalopathy.
Patient 2
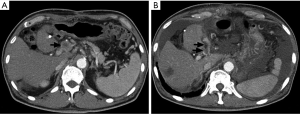
A 66-year-old man with jaundice and appetite loss was admitted to our hospital. He had been diagnosed with type-2 diabetes mellitus more than 10 years earlier. Fifteen months before admission, an HCC 8 cm in diameter was found in the left lobe of his liver. Although he underwent left lobe resection, owing to his good hepatic functional reserve, intrahepatic tumor recurrences were repeatedly observed. He underwent two sessions of transcatheter arterial chemoembolization (TACE), 11 and 7 months before admission. After the second TACE session, several lymph node (LN) metastases close to the portal vein were found, whereas no intrahepatic recurrences were observed. As radiation therapy was ineffective in treating the metastases, sorafenib (400 mg/day) treatment was subsequently started while the patient was hospitalized. Adverse events included hand-foot skin reaction, an increase of BP, and an increase in of LDH level (221 to 277 U/L). A limited blood clot thrombosis in the portal vein trunk, observed prior to sorafenib therapy, was not regarded as an obstacle to treatment. He was discharged from the hospital 7 days after sorafenib treatment, followed by continuous administration of 400 mg/day sorafenib. Seven days later, he experienced jaundice and progressive loss of appetite. Blood tests on admission showed considerable deterioration of liver function, including PT-INR of 4.97, albumin 2.4 g/dL, ALT of 777 U/L, LDH of 393 U/L, total bilirubin of 18.6 mg/dL, and direct bilirubin of 12.2 mg/dL. Doppler ultrasonography and CT examination showed the extension of portal thrombosis to the intrahepatic branches and the appearance of moderate ascites (Figure 3). Despite efforts to support liver function with plasma exchange, his liver size rapidly decreased, leading to fatal liver failure in 1 week.
Discussion
The anti-cancer activity of sorafenib is thought to be due to two different pathways: inhibition of the serine-threonine kinase Raf-1, which decreases mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated kinase (ERK) activity, resulting in the suppression of tumor cell proliferation; and the inhibition of kinases associated with angiogenesis, such as vascular endothelial growth factor (VEGF) receptors-2 and -3 and platelet-derived growth factor receptor β (18). As sorafenib treatment has become more widespread, so has the number of these patients experiencing increases in BP during sorafenib treatment (19). Furthermore, several patients have developed coronary artery spasm during sorafenib therapy (20-22). These adverse effects may be due to the inhibition by sorafenib of the VFGF signaling pathway, decreasing endothelial nitric oxide synthase (eNOS) expression by endothelial cells and causing vascular smooth muscle cell constriction (23). These phenomena may have played key role in the rapid development of liver failure observe in our patients.
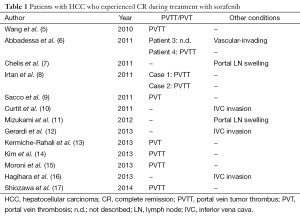
In considering the mechanism of liver failure in our patients, we found characteristics in common with previously reported patients with HCC who achieved CR on sorafenib monotherapy (5-17). Of 15 patients, seven had portal vein tumor thrombi, two had portal thrombosis, three had tumor invasion into the inferior vena cava (IVC), two had portal LN swelling, and one had vascular-invading HCC (Table 1). Taken together, all of these patients had a condition that could decrease portal blood flow, strongly indicating that deterioration in portal flow is essential to achieving CR on sorafenib monotherapy. That is, sorafenib can induce CR in patients with HCC by compressing the hepatic artery following decreased portal flow, a process that could cause extreme hypoxic circumstances in the liver.
Full table
Although liver hypoxia may be indispensable for CR, the decrease of both portal and arterial blood flow might also result in liver failure. Indeed, sorafenib was found to induce partial remission in two patients with HCC, followed by the rapid development of liver failure (24). Because sorafenib administration to patients with decreased portal flow could cause both CR and liver failure, two biomarkers of hypoxia, LDH and DCP, should be monitored during treatment with sorafenib (25,26). Better prognosis was observed in patients with low than high pretreatment LDH levels who were treated with sorafenib (25). In contrast, time to tumor progression was significantly longer in patients with high than low DCP concentrations during treatment with sorafenib (26). These conflicting results suggest that, although sorafenib-induced liver hypoxia can suppress tumor growth, it could also adversely affect prognosis when hepatic functional reserve is insufficient, an effect amplified in patients with reduced portal flow.
Patient 1 showed a marked increase in LDH on the day he developed hepatic encephalopathy, indicating that his liver was in a hypoxic condition. The simultaneous elevation in BP suggested the constriction of the systemic vascular endothelial cells. Cessation of sorafenib treatment effected a rapid improvement in encephalopathy and decreases in serum LDH and ammonia levels. The prompt reversibility of these conditions suggested that acute liver failure in this patient was due to vascular constriction, not massive hepatocyte death. The findings in patient 2 suggest that PVT, in addition to PVTT, could induce acute liver failure and that prolonged intrahepatic hypoxia could cause fatal liver failure. Patient 2 experienced portal thrombosis before sorafenib treatment, with elevated LDH concentration observed 7 days after the start of sorafenib monotherapy. Blood examination was not performed until 1 month later, when the portal thrombus had extended and his liver failure had fatally progressed. If the risk of liver hypoxia in the presence of portal thrombosis had been perceived and had been more closely observed, liver failure may have been avoided.
In conclusion, sorafenib treatment of patients with HCC and deteriorated portal flow may be a double-edged sword. Although it may lead to CR, it may also result in liver failure. Caution should be exercised in treating patients with sorafenib, especially those with inadequate hepatic functional reserve.
Acknowledgements
The authors would like to thank Ms. Keiko Nishimura for editorial support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Woo HY, Heo J, Yoon KT, et al. Clinical course of sorafenib treatment in patients with hepatocellular carcinoma. Scand J Gastroenterol 2012;47:809-19. [Crossref] [PubMed]
- Wörns MA, Weinmann A, Pfingst K, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol 2009;43:489-95. [Crossref] [PubMed]
- Wang SX, Byrnes A, Verma S, et al. Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol 2010;5:59-63. [Crossref] [PubMed]
- Abbadessa G, Rimassa L, Pressiani T, et al. Optimized management of advanced hepatocellular carcinoma: four long-lasting responses to sorafenib. World J Gastroenterol 2011;17:2450-3. [Crossref] [PubMed]
- Chelis L, Ntinos N, Souftas V, et al. Complete response after sorafenib therapy for hepatocellular carcinoma in an HIV-HBV co infected patient: Possible synergy with HAART ? A case report. Med Oncol 2011;28 Suppl 1:S165-8. [Crossref] [PubMed]
- Irtan S, Chopin-Laly X, Ronot M, et al. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int 2011;31:740-3. [Crossref] [PubMed]
- Sacco R, Bargellini I, Gianluigi G, et al. Complete response for advanced liver cancer during sorafenib therapy: case report. BMC Gastroenterol 2011;11:4. [Crossref] [PubMed]
- Curtit E, Thiery-Vuillemin A, Nguyen T, et al. Complete histologic response induced by sorafenib in advanced hepatocellular carcinoma: a case report. J Clin Oncol 2011;29:e330-2. [Crossref] [PubMed]
- Mizukami H, Kagawa T, Arase Y, et al. Complete response after short-term sorafenib treatment in a patient with lymph node metastasis of hepatocellular carcinoma. Case Rep Oncol 2012;5:380-4. [Crossref] [PubMed]
- Gerardi AM, Stoppino LP, Liso A, et al. Rapid long-lasting biochemical and radiological response to sorafenib in a case of advanced hepatocellular carcinoma. Oncol Lett 2013;5:975-977. [PubMed]
- Kermiche-Rahali S, Di Fiore A, Drieux F, et al. Complete pathological regression of hepatocellular carcinoma with portal vein thrombosis treated with sorafenib. World J Surg Oncol 2013;11:171. [Crossref] [PubMed]
- Kim MS, Jin YJ, Lee JW, et al. Complete remission of advanced hepatocellular carcinoma by sorafenib: A case report. World J Gastrointest Oncol 2013;5:38-42. [PubMed]
- Moroni M, Zanlorenzi L. Complete regression following sorafenib in unresectable, locally advanced hepatocellular carcinoma. Future Oncol 2013;9:1231-7. [Crossref] [PubMed]
- Hagihara A, Teranishi Y, Kawamura E, et al. A complete response induced by 21-day sorafenib therapy in a patient with advanced hepatocellular carcinoma. Intern Med 2013;52:1589-92. [Crossref] [PubMed]
- Shiozawa K, Watanabe M, Ikehara T, et al. Sustained complete response of hepatocellular carcinoma with portal vein tumor thrombus following discontinuation of sorafenib: A case report. Oncol Lett 2014;7:50-52. [PubMed]
- Murphy DA, Makonnen S, Lassoued W, et al. Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006). Am J Pathol 2006;169:1875-85. [Crossref] [PubMed]
- Li Y, Li S, Zhu Y, et al. Incidence and risk of sorafenib-induced hypertension: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2014;16:177-85. [Crossref] [PubMed]
- Porto I, Leo A, Miele L, et al. A case of variant angina in a patient under chronic treatment with sorafenib. Nat Rev Clin Oncol 2010;7:476-80. [Crossref] [PubMed]
- Pantaleo MA, Mandrioli A, Saponara M, et al. Development of coronary artery stenosis in a patient with metastatic renal cell carcinoma treated with sorafenib. BMC Cancer 2012;12:231. [Crossref] [PubMed]
- Arima Y, Oshima S, Noda K, et al. Sorafenib-induced acute myocardial infarction due to coronary artery spasm. J Cardiol 2009;54:512-5. [Crossref] [PubMed]
- Humphreys BD, Atkins MB. Rapid development of hypertension by sorafenib: toxicity or target? Clin Cancer Res 2009;15:5947-9. [Crossref] [PubMed]
- Nakazawa T, Hidaka H, Shibuya A, et al. Rapid regression of advanced hepatocellular carcinoma associated with elevation of des-gamma-carboxy prothrombin after short-term treatment with sorafenib - a report of two cases. Case Rep Oncol 2010;3:298-303. [Crossref] [PubMed]
- Faloppi L, Scartozzi M, Bianconi M, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer 2014;14:110. [Crossref] [PubMed]
- Ueshima K, Kudo M, Takita M, et al. Des-γ-carboxyprothrombin may be a promising biomarker to determine the therapeutic efficacy of sorafenib for hepatocellular carcinoma. Dig Dis 2011;29:321-5. [Crossref] [PubMed]


