Use of molecular studies for treatment of metastatic pleomorphic large cell pancreatic cancers—a novel strategy
Introduction
Pancreatic cancer is the 4th leading cause of cancer death in the United States and there are approximately 46,000 new cases per year (1). Unfortunately outcomes of metastatic pancreatic adenocarcinoma have been dismal. Over the last 3-4 years new treatment regimens have been studied but the median overall survival is between 8.5-11.1 months (2,3). Pleomorphic large cell pancreatic cancer is a rare and more aggressive variant with no proven treatment in the metastatic setting. There have been variable outcomes in patients who are deemed surgically resectable (4,5). Now with the advent of molecular profiling by immunohistochemistry, next-generation sequencing and chromogenic in-situ hybridization we are able to study these cancers at a molecular level in order to make chemotherapeutic decisions. We are presenting a case where the use of molecular profiling assisted us in treatment as well as better understanding the disease of undifferentiated pleomorphic large cell pancreatic cancer. Our case is one of the earliest documented cases of using molecular profiling to aid in the treatment of this rare entity and it will shed light in managing patients with this rare cancer.
Case presentation
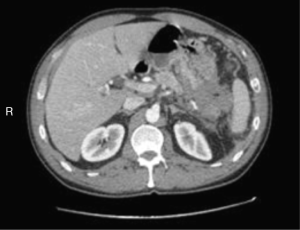
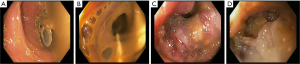
We are presenting a 50-year-old Caucasian male who presented to our hospital in October 2014. He has no significant past medical history except for daily alcohol and tobacco use. He had been complaining of episodic abdominal pain that has been progressively getting worse. Two months prior he had a CT scan of his abdomen that suggested cholelithiasis, for which he underwent elective laparoscopic cholecystectomy. The pain persisted despite the surgery and he presented to an outside facility. A repeat CT scan of the abdomen revealed a 4 cm × 1.9 cm ill-defined pancreatic mass that appeared to infiltrate the splenic flexure of the colon and gastric fundus (Figure 1). He underwent a colonoscopy that revealed a fungating mass eroding into the splenic flexure causing a complete obstruction (Figure 2). Hence an EGD and EUS were performed and a Wallstent SEMS was placed across the obstruction. EUS also revealed a large pancreatic tail mass from which biopsies were taken, Pathology showed areas of chronic inflammation and atypical cells but no malignant cells were identified. He eventually underwent an exploratory laparotomy with biopsy of the omentum, pareito-peritoneum and placement of a transverse loop colostomy. During the surgery, there were findings of studding of the parietoperitoneum in the right and lower upper quadrant. In addition, the poorly defined mass involved segments of the transverse colon, splenic flexure, distal pancreas, spleen, the posterior body of the stomach, the greater curvature.
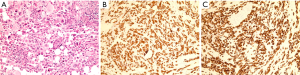
Pathology from the omental and peritoneal nodules revealed an undifferentiated carcinoma with pleomorphic giant cells as well as malignant spindle and sarcomatoid features. The neoplastic cells were positive for CAM 5.2, EMA, CK7 and negative for CK20, TTF-1, CDX-2, CK5/6, P63, PSAP, RCC and PAX-8 suggestive of a pancreatic origin (Figure 3).
On reviewing the literature; pleomorphic undifferentiated pancreatic cancers are a rare variant of pancreatic cancers with no standard regimens for treating metastatic disease. It’s an aggressive variant having poor outcomes. Hence we sent his specimen for molecular profile and mutation analysis to better understand his disease and also to assist us in his management.
The molecular studies revealed that his malignancy was positive for mutations of TLE3 gene, EGFR, KRAS, PD1 gene, TP53 and TOP2A gene and was negative for Her2Neu, RRM1, BRAF, RET, PI3KCA, BRCA and MEK. It also revealed that the tumor was most sensitive to gemcitabine, paclitaxel, docetaxel, temozolamide, dacarbazine and doxorubicin. The platinum agents, MTOR inhibitors and TKIs had indeterminate to no benefit.
After discussion of his case and reviewing the molecular data he was initiated with gemcitabine (1,000 mg/m2) and nab-paclitaxel (125 mg/m2). He tolerated the chemotherapy well. Unfortunately after 5 cycles he had developed renal failure requiring temporary dialysis, hemolytic anemia and thrombocytopenia leading to cessation of chemotherapy. He was diagnosed with gemcitabine induced atypical hemolytic uremic syndrome which was biopsy proven. Eculizumab was initiated, and he has had significant improvement in his hematological parameters and renal function. Serial CT scans have shown stable disease and currently it has been 10 months since his diagnosis. In the event that he does progress it would be challenging to decide the chemotherapy regimen for him, given the toxicity from Gemcitabine.
Discussion
Undifferentiated pancreatic malignancies are rare variants of exocrine pancreatic malignancies. For the most part it has an aggressive course. It makes up about 1% of all pancreatic malignancies (4). Undifferentiated cancers are divided into two major variants, osteoclastic giant cell tumors and pleomorphic large cell cancers. The latter being highly proliferative (6).
Sommer and Meisner were the first to recognize this rare variant of pancreatic cancer. It was initially described as “bizzare pancreatic tumor characterized by giant cells and sarcomatoid features.” This led to it being called pancreatic sarcoma (7). There has been controversy as to the origin of these cells. With the advancement in molecular testing and the availability of immunohistochemistry, differentiating between these subtypes has made some headway over the years. Review of the IH patterns published in the recent past shows that osteoclast-like giant cells of OGCT are positive for mesenchymal markers, whereas the pleomorphic giant cells of PGCT are strongly positive for epithelial markers. The neoplastic cells of PGCT show positive staining for cytokeratin 7 and cytokeratin 19 (8,9). In our patient, the IH showed staining positive for CAM 5.5 and CK 7 and negative for CK20, CDX-2, and p-63, PSA, PSAP, TTF-1, PAX-8 and CK5/6.
Undifferentiated cancers do stain for cytokeratin 7, 8, 18 and 19 and have high staining for Ki-67 thus there is a possibility that they do arise from the ductal cells as well (10,11). Pleomorphic pancreatic cancers tend to have mutations of KRAS and P53.
These cancers arise from the body and tail of the pancreas, in most cases. It affects patients during the 6th and 7th decade of life (12). Most of the tumors are greater than 6 cm without vascular invasion (13). The symptoms range from abdominal pain to causing bowel obstruction/perforation given their size and location. Given the rarity of this disease there are no clinical trials to determine the chemotherapeutic agents that would benefit these patients. There have been case series regarding the osteoclastic variant of undifferentiated cancer that have been surgically resected with variable results. One case series had most patients that died within the first year (14). Another case series with resectable anaplastic (undifferentiated) cancers showed a median overall survival of 5.7 months. In the cases osteoclastic giant cell histology there has been overall survival of greater than 2 years (15), five years, and in a few reports more than fifteen years (16). 5-fluorouracil and gemcitabine have been used in recurrent/non-operable osteoclastic giant cell tumors without good success (17).
Today, with the advent of molecular testing it can be instrumental to send pathological samples for analysis. Testing may assist us in choosing agents that will be the most beneficial for the patient. Identifying driver mutations in rare malignancies will help in finding target agents. KRAS mutations, loss of function of P16/CDKN2A, TP53 and SMAD/DPC4 genes (18) are few of the driver mutations in pancreatic malignancies. In our case, the tumor was positive for EGFR, KRAS, TL3, TOP3A, PD1 and TP53 genes. TOP3A mutations are seen in breast cancers especially in BRCA positive malignancies (19). The future of treatment of metastatic pancreatic cancer depends on inhibiting driver mutations. Use of farnesyltransferase inhibitors such as tipifarnib and lonafarnib have shown impressive anti-tumor activity and anti-RAS activity in preclinical cell cultures as well as mouse embryos (20,21). Downstream to the RAS pathway are the mTOR, PI3K and MEK pathways, all of which can be targeted (22). The current HALO 109-202 trial (23) using hyaluronidase to induce an immune response to metastatic pancreatic ductal cancer is currently ongoing. The future is bright and hopefully we can see benefit of immune therapy and targeted molecular therapy as the future of pancreatic cancer management.
Conclusions
Large cell pancreatic cancer is a rare form of neoplasm with histology and clinical symptoms unique to its kind. Due to the paucity of cases reported and lack of a standard treatment outlined for their management, it is important to follow up these patients over an extended period of time so as to aid in the compilation of literature to guide the further research and forming a guideline for the treatment of this peculiar form of cancer. The future of treatments depends on the overall and disease free survival benefit we can obtain by targeted and immunotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society, 2014.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [PubMed]
- Temesgen WM, Wachtel M, Dissanaike S. Osteoclastic giant cell tumor of the pancreas. Int J Surg Case Rep 2014;5:175-9. [PubMed]
- Hur YH, Kim HH, Seoung JS, et al. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. J Korean Surg Soc 2011;81:146-50. [PubMed]
- Compton CC. Atlas of Tumor Pathology. Tumors of the Pancreas. Gastroenterology 1998;114:614.
- Sommers SC, Meissner WA. Unusual carcinoma of the pancreas. AMA Arch Pathol 1954;58:101-11. [PubMed]
- Tschang TP, Garza-Garza R, Kissane JM. Pleomorphic carcinoma of the pancreas: an analysis of 15 cases. Cancer 1977;39:2114-26. [PubMed]
- Trepeta RW, Mathur B, Lagin S, et al. Giant cell tumor ("osteoclastoma") of the pancreas: a tumor of epithelial origin. Cancer 1981;48:2022-8. [PubMed]
- Verbeke CS, Menon KV. Osteoclast-like giant cell tumour of the pancreas: an undifferentiated carcinoma of duct epithelial origin. Pancreatology 2006;6:254; author reply 254. [PubMed]
- Hoorens A, Prenzel K, Lemoine NR, et al. Undifferentiated carcinoma of the pancreas: analysis of intermediate filament profile and Ki-ras mutations provides evidence of a ductal origin. J Pathol 1998;185:53-60. [PubMed]
- Rustagi T, Rampurwala M, Rai M, et al. Recurrent acute pancreatitis and persistent hyperamylasemia as a presentation of pancreatic osteoclastic giant cell tumor: an unusual presentation of a rare tumor. Pancreatology 2011;11:12-5. [PubMed]
- Tezuka K, Yamakawa M, Jingu A, et al. An unusual case of undifferentiated carcinoma in situ with osteoclast-like giant cells of the pancreas. Pancreas 2006;33:304-10. [PubMed]
- Molberg KH, Heffess C, Delgado R, et al. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer 1998;82:1279-87. [PubMed]
- Strobel O, Hartwig W, Bergmann F, et al. Anaplastic pancreatic cancer: Presentation, surgical management, and outcome. Surgery 2011;149:200-8. [PubMed]
- Suda K, Takase M, Oyama T, et al. An osteoclast-like giant cell tumor pattern in a mucinous cystadenocarcinoma of the pancreas with lymph node metastasis in a patient surviving over 10 years. Virchows Arch 2001;438:519-20. [PubMed]
- Singhal A, Shrago SS, Li SF, et al. Giant cell tumor of the pancreas: a pathological diagnosis with poor prognosis. Hepatobiliary Pancreat Dis Int 2010;9:433-7. [PubMed]
- Korc M. Driver mutations: a roadmap for getting close and personal in pancreatic cancer. Cancer Biol Ther 2010;10:588-91. [PubMed]
- Broberg K, Huynh E, Schläwicke Engström K, et al. Association between polymorphisms in RMI1, TOP3A, and BLM and risk of cancer, a case-control study. BMC Cancer 2009;9:140. [PubMed]
- Sepp-Lorenzino L, Ma Z, Rands E, et al. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res 1995;55:5302-9. [PubMed]
- James G, Goldstein JL, Brown MS. Resistance of K-RasBV12 proteins to farnesyltransferase inhibitors in Rat1 cells. Proc Natl Acad Sci U S A 1996;93:4454-8. [PubMed]
- Baines AT, Xu D, Der CJ. Inhibition of Ras for cancer treatment: the search continues. Future Med Chem 2011;3:1787-808. [PubMed]
- Clinical trials.gov. PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Subjects With Stage IV Untreated Pancreatic Cancer (HALO-109-202). Available online: https://clinicaltrials.gov/ct2/show/NCT01839487