
Locally advanced anal small cell carcinoma with durable complete response to chemoradiation followed by consolidation chemotherapy: case report and literature review
Introduction
Anal cancer is an uncommon malignancy of which the majority are squamous cell cancers that have well-established treatments and overall favorable prognosis (1). However, primary small cell carcinoma of the anus is an extremely rare and aggressive clinical entity with poorly defined treatment and unfavorable prognosis. In this case report and literature review, we report a patient with locally advanced small cell carcinoma of the anus with a durable complete response after treatment with concurrent cisplatin and etoposide with radiation therapy followed by four cycles of consolidation chemotherapy with cisplatin and etoposide. Given the overall favorable and durable response to treatment with chemoradiation and consolidation chemotherapy, this treatment strategy may represent a new paradigm for the management of this rare and poorly understood disease. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-434).
Case presentation
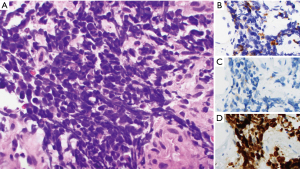
A 66-year-old female with no significant past medical or family history initially presented with irregular bowel movements, changes in stool caliber, and rectal bleeding for two months prior to presentation (Table 1). Initial physical exam demonstrated a large, indurated mass in the anus encompassing the anal sphincter muscle. A colonoscopy was subsequently performed, which identified a partially obstructing, friable, partially circumferential mass starting at the dentate line and extending into the mid rectum. A biopsy of the mass demonstrated histomorphologic and immunophenotypic features consistent with small cell neuroendocrine carcinoma (Figure 1).
Table 1
| Significant clinical events | Dates |
|---|---|
| Initial presentation | 7/24/2018 |
| Chemoradiation with cisplatin and etoposide | 9/17/2018–10/22/2018 |
| Cycle 1 of consolidation chemotherapy with cisplatin and etoposide | 11/23/2018–11/25/2018 |
| Cycle 2 of consolidation chemotherapy with cisplatin and etoposide | 12/14/2018–12/16/2018 |
| Cycle 3 of consolidation chemotherapy with cisplatin and etoposide | 1/4/2018–1/6/2018 |
| Cycle 4 of consolidation chemotherapy with cisplatin and etoposide | 1/25/2019–1/27/2019 |
| Surveillance | 2/15/2019–present. Last follow-up date on 3/17/2021 |
Initial laboratory studies were largely unremarkable except a normocytic anemia with hemoglobin of 10.5 g/dL. Staging computerized tomography (CT) studies of the chest, abdomen, and pelvis demonstrated a 6×4×8 cm3 mass in the anus with associated multiple enlarged left inguinal lymph nodes. A subsequent MRI of the abdomen and pelvis along with 18F-FDG PET scan confirmed a large 12.7×7.9×5.9 cm3 mass in the anus associated with an enlarged 1.3 cm peri-rectal lymph node; no distant metastases were identified (Figure 2).
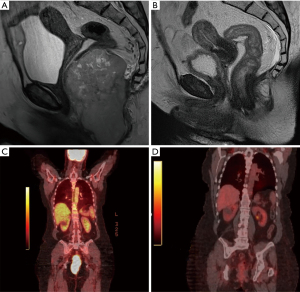
The patient was treated with definitive concurrent chemoradiation with cisplatin 50 mg/m2 on days 1, 8, 29, 36 and etoposide 50 mg/m2 on days 1–5 and 29–33. She received a total dose of 59 Gy in 32 fractions (45 Gy in 1.8 per fraction followed by boost RT to gross tumor at the anal canal for 14 Gy in 2 Gy per fraction) using helical tomotherapy to cover the anorectal canal and the regional lymph nodes, including mesorectum, bilateral inguinal, external and internal iliac, and common iliac lymph nodes. Within a few weeks of starting treatment, the patient developed marked improvement in pain and bowel movement frequency. No significant adverse reactions or unanticipated events from therapy were reported. Two weeks after completion of therapy CT scans of the chest and MRI of the abdomen and pelvis demonstrated a significant response to treatment with a decrease in size of mass to 1.2×0.8×1.1 cm3 from 12.7×7.9×5.9 cm3. MRI of the brain did not show evidence of brain metastases.
Given concern for high risk of relapse, the patient was treated with an additional four cycles of consolidation chemotherapy with cisplatin 75 mg/m2 on day 1 and etoposide 100 mg/m2 on days 1–3 every 21 days four weeks after completion of chemoradiation. Six weeks after completion of treatment, clinical examination and flexible sigmoidoscopy demonstrated complete regression of the anal tumor. Repeat imaging with CT of the chest and MRI of the abdomen and pelvis along with 18F-FDG PET confirmed a complete response to therapy. Nearly 36 months from initial diagnosis, the patient continues to have no clinical or radiographic evidence of disease recurrence and remains on close surveillance which entails a history and physical examination including a rectal exam with PET/CT imaging every 6 months.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the City of Hope institutional review board and with the Helsinki Declaration (as revised in 2013). This study was conducted under an Institutional Review Board approved protocol (IRB14361). Because this was an outcome analysis study and the case was de-identified, the Institutional Review Board did not require an informed consent.
Methods
We discuss a rare case of anal small cell carcinoma which initially presented with irregular bowel movements, changes in stool caliber, and rectal bleeding along with its management and treatment outcomes. We also performed a literature search using MEDLINE to identify clinical studies (including case reports or series and any observation, retrospective studies) that discussed cases of patients with anal small cell carcinoma along with their management and overall outcomes to treatment. The search was limited by the following key words: anal small cell carcinoma, extrapulmonary small cell carcinoma (EPSCC), and treatment outcomes. A manual search was also performed to include additional studies of potential relevance.
Discussion
Small cell carcinoma is a high-grade poorly differentiated malignancy with neuroendocrine differentiation. It is an aggressive clinical entity that commonly arises in the lungs. However, extrapulmonary small cell carcinoma (EPSCC) is well-recognized and known to frequently occur in the genitourinary, gynecologic, and gastrointestinal tract (2,3). Each year approximately 1,000 new cases of EPSCC are diagnosed, representing 0.1% to 0.4% of all cancers diagnosed in the United States and 2.5 to 5% of all small cell carcinoma Prognosis for EPSCC is poor with a median overall survival (OS) of 1.2 years with recent data suggesting that anatomic location influences clinical outcomes. EPSCC involving the gastrointestinal is associated with the worse prognosis while EPSCC involving the head and neck, and breast have improved overall survival (3-5). The impact of anatomic location on outcomes is postulated to be related to the effect on the timing of the initial presentation.
Of the cases involving the gastrointestinal tract, the majority of reported cases involve the esophagus, colon, and rectum (6). Small cell carcinoma of the anal tract is extremely rare, and the true incidence of this entity is not known. The predominant discussion of this disease in the literature has been largely limited to a handful of case reports (Table 2) (7-15). The majority of the patients were treated with a multimodality approach consisting of chemotherapy with cisplatin and etoposide along with radiation therapy. Surgery was commonly used as salvage therapy for local recurrence. Many patients had rapid disease progression and nearly all patients had an overall survival of less than 12 months after diagnosis. While there is a strong association between select high-risk subtypes of human papillomavirus (HPV) and HIV with the development of squamous cell carcinoma of the anus, the risk factors for the development of anal small cell carcinoma are not well established. However, several cases of anal small cell carcinoma have been reported to develop in patients with both HIV and HPV (7,9,13,16).
Table 2
| Author (year) | Clinical presentation | Treatment | Clinical outcome | Citation |
|---|---|---|---|---|
| Nakahara [1993] | 48-year-old male with HIV found to have localized anal small cell carcinoma | Initially treated with radiation with complete response however treated with surgery and adjuvant chemotherapy with cisplatin 80 mg/m2 on day 1 and etoposide 75 mg/m2 on days 1–3 after local recurrence in pelvic cavity | Patient committed suicide 11 weeks after surgery. No disease recurrence after surgery and adjuvant chemotherapy | (7) |
| Meyer [2007] | 41-year-old woman with metastatic anal small cell carcinoma with liver and lungs metastasis | Initially treated with 6 cycles of cisplatin 45 mg/m2 on days 2 and 3 and etoposide 130 mg/m2 on day 1 repeated every 3 weeks with pelvic irradiation for local pelvic recurrence | Disease progression followed by death 10 months after diagnosis | (8) |
| Alcindor [2008] | 45-year-old male with HIV found to have anal small cell carcinoma with liver metastases | Chemoradiation with cisplatin and etoposide | Progression of disease after initial response to therapy; deceased 6 months after diagnosis | (9) |
| Doddi [2009] | 60-year-old female with localized anal small cell carcinoma | Chemotherapy with cisplatin and etoposide followed by radiation therapy | Clinical relapse 12 months after completion of therapy; deceased 18 months after initial diagnosis | (10) |
| Khan [2009] | 50-year-old male with locally advanced anal small cell carcinoma | Initial treatment with chemoradiation with cisplatin and etoposide; surgery and repeat chemoradiation with cisplatin/irinotecan for local recurrence. Repeat radiation non-overlapping with initial fields. Third line topotecan for bony recurrence | Complete response to chemoradiation followed by local recurrence 24 months after completion of initial therapy. Patient achieved another 24 months of disease-free after repeat chemoradiation interval prior to developing bony recurrence | (11) |
| Eberhardt [2012] | 63-year-old female with anal small cell carcinoma with involvement of right inguinal lymph node | Chemoradiation with cisplatin and etoposide | Progression of disease 3 months after completion of therapy; deceased 10 months after diagnosis | (12) |
| Marcus [2013] | 49-year-old male with HIV found to have anal small cell carcinoma with involvement of perirectal and inguinal lymph nodes | Chemoradiation with cisplatin 80 mg/m2 and etoposide 100 mg/m2 days 1–3 every 21 days | Complete response to chemoradiation. No evidence of disease recurrence at 5-month follow-up post-treatment | (13) |
| Khmou [2014] | 53-year-old male with locally advanced anal small cell carcinoma | Chemoradiation | Deceased 12 months after diagnosis | (14) |
| Surag [2016] | 46-year-old-male with locally advanced anal small cell carcinoma with involvement of inguinal, mesorectal and internal iliac lymph nodes | Surgery followed by adjuvant chemotherapy with cisplatin and etoposide | Disease progression while on chemotherapy; deceased 4 months after diagnosis | (15) |
Small cell carcinoma of the gastrointestinal tract commonly presents with widespread dissemination (3). Even patients who initially present with locoregional involvement progress rapidly to systemic disease (17). Given the rarity of EPSCC, there are no prospective trials to guide management. Treatment of EPSCC has been largely extrapolated from the treatment of small cell carcinomas from other anatomic locations, namely small cell lung carcinoma, which favors concurrent chemotherapy with radiation therapy. A single-center retrospective study of 120 patients with predominantly non-metastatic EPSCC had comparable survival to patients with limited disease small cell lung cancer treated at the same institution (2). Another retrospective study of 5,747 patients with EPSCC from the National Cancer Database (NCDB) between 2006–2014 found that concurrent chemotherapy and radiation demonstrated improved overall survival to chemotherapy or radiation alone (3). These findings have led to the adoption of the treatment approach for small cell lung carcinoma to the management of EPSCC. While small cell carcinoma of the lungs has a predilection for central nervous system involvement, EPSCC has been shown to have a lower risk of CNS metastases (2,18,19). Therefore, prophylactic cranial irradiation has not been widely adopted in the treatment of EPSCC.
The case we discuss represents the only anal small cell carcinoma case we are aware of with a durable response nearly 36 months after diagnosis. While objective responses to concurrent chemotherapy and radiation are commonly observed, nearly all cases relapsed within 12 months of diagnosis, usually after treatment with concurrent chemotherapy and radiation. Given the high risk of relapse, the addition of consolidation systemic therapy to concurrent chemoradiation in our case likely further decreased the risk of relapse in our patient and prolonged overall survival. Furthermore, in contrast to previous cases reported, we administered a protocol of cisplatin and etoposide that is more commonly seen in the treatment of non-small cell cancer, which concurrently with pelvic irradiation is feasible and well tolerated. Therefore, this novel approach to management should be strongly considered for treatment of this rare and aggressive disease.
In conclusion, small cell anal carcinoma is a rare and poorly understood entity with sparse data to guide management of this aggressive disease. Risk of relapse is high despite treatment with concurrent chemotherapy and radiation. An aggressive approach to management with the addition of consolidation chemotherapy to concurrent chemoradiation may offer prolonged disease-free survival and improved overall survival. Further studies will be required to delineate the risk factors for developing this disease and better define the treatment approach for this rare entity.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-434
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-434). Dr. MF has consulted for HalioDx, Mirati, Pfizer, Taiho, and Zhuhai Yufan Biotech. He has served on Advisory Boards for Amgen, Array Biopharma, Bayer, GSK, Mirati and Seattle Genetics. He has received Honoraria or participated in Speaker Bureaus for Amgen and Guardant Health. Dr. VP is a consultant for Genentech. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the City of Hope institutional research board research committee(s) and with the Helsinki Declaration (as revised in 2013). This study was conducted under an Institutional Review Board approved protocol (IRB14361). Because this was an outcome analysis study and the case was de-identified, the Institutional Review Board did not require an informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med 2000;342:792-800. [Crossref] [PubMed]
- Brennan SM, Gregory DL, Stillie A, et al. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer 2010;116:888-95. [Crossref] [PubMed]
- Mandish SF, Gaskins JT, Yusuf MB, et al. Extrapulmonary small cell carcinoma: Prognostic factors, patterns of care, and overall survival. Eur J Surg Oncol 2020;46:1596-604. [Crossref] [PubMed]
- Wong YN, Jack RH, Mak V, et al. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970-2004. BMC Cancer 2009;9:209. [Crossref] [PubMed]
- Brammer JE, Lulla P, Lynch GR. Retrospective review of extra-pulmonary small cell carcinoma and prognostic factors. Int J Clin Oncol 2014;19:822-8. [Crossref] [PubMed]
- Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev 2009;35:228-36. [Crossref] [PubMed]
- Nakahara H, Moriya Y, Shinkai T, et al. Small cell carcinoma of the anus in a human HIV carrier: report of a case. Surg Today 1993;23:85-8. [Crossref] [PubMed]
- Meyer A, Bruns F, Richter K, et al. Small cell cancer of the anal canal--case report of a rare tumor. Anticancer Res 2007;27:1047-50. [PubMed]
- Alcindor T, Tosikyan A, Vuong T, et al. Small-cell anal carcinoma and AIDS: case report and review of the literature. Int J Colorectal Dis 2008;23:135-6. [Crossref] [PubMed]
- Doddi S, Singhal T, De Silva C, et al. Small cell carcinoma of the anus: a case report. Cases J 2009;2:9396. [Crossref] [PubMed]
- Khan GN, Siddiqui M, Ben-Josef E, et al. An unusual case of small cell carcinoma of the anal canal. Am J Clin Oncol 2009;32:543-4. [Crossref] [PubMed]
- Eberhardt JM, Brown K, Lo S, et al. Extrapulmonary small cell carcinoma of the anal canal: a case report and review of the literature. Case Rep Med 2012;2012:341432. [Crossref] [PubMed]
- Marcus DM, Edgar MA, Hawk NN, et al. Small cell carcinoma of the anus in the setting of prior squamous dysplasia and carcinoma in situ. J Gastrointest Oncol 2013;4:E1-4. [PubMed]
- Khmou M, Zouaidia F, Ouazzani H, et al. Small-cell carcinoma of the anal canal: Case report of a rare tumor. African Journal of Cancer 2014;6:186-8. [Crossref]
- Surag Neeralagi C. Small Cell Carcinoma of Anal Canal - A Rare Case Report. J Clin Diagn Res 2016;10:PD14-5. [PubMed]
- Cimino-Mathews A, Sharma R, Illei PB. Detection of human papillomavirus in small cell carcinomas of the anus and rectum. Am J Surg Pathol 2012;36:1087-92. [Crossref] [PubMed]
- Boman BM, Moertel CG, O'Connell MJ, et al. Carcinoma of the anal canal. A clinical and pathologic study of 188 cases. Cancer 1984;54:114-25. [Crossref] [PubMed]
- Cicin I, Karagol H, Uzunoglu S, et al. Extrapulmonary small-cell carcinoma compared with small-cell lung carcinoma: a retrospective single-center study. Cancer 2007;110:1068-76. [Crossref] [PubMed]
- Soto DE, Eisbruch A. Limited-stage extrapulmonary small cell carcinoma: outcomes after modern chemotherapy and radiotherapy. Cancer J 2007;13:243-6. [Crossref] [PubMed]


