
Ovarian transposition and metachronous ovarian metastasis in a premenopausal colorectal carcinoma patient: a case report
Introduction
Colon cancer has a high incidence of metastasis, with 20% of patients presenting with Stage IV disease at diagnosis (1). Common sites of metastasis include the lymph nodes, liver, lungs, and peritoneum. An estimated 0.8–7.4% of colorectal adenocarcinoma (CRC) cases metastasize to the ovary, with even higher rates discovered postmortem at 5–9.7% (2). Additionally, an estimated 43–70% of ovarian metastases are bilateral (3). Metastases to the ovary are associated with aggressive disease and poor outcomes (4). For young women with colon cancer, available treatment options often impact fertility. Even small doses of radiation to the ovary can effectively sterilize premenopausal women, with estimates of just 14.3 Gy inducing complete ovarian failure, and radiation tolerances for the ovary limited to just 2 Gy (5). Ovarian transposition is a surgical approach to limit ovarian radiation exposure and preserve fertility. However, it is contested whether ovarian preservation in reproductive age women with colon cancer is advantageous or presents a significant risk for disease recurrence and subsequent morbidity and mortality.
We present a case report of a young female with locally advanced colon cancer who underwent transposition of the contralateral ovary and subsequently experienced metachronous metastasis post chemoradiation and a discussion of the role of ovarian preservation versus prophylactic bilateral oophorectomy. Our case is unique in addressing a premenopausal patient with fertility concerns, for whom the consequences of unnecessary oophorectomy, or of undertreated malignancy, are particularly devastating. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-558).
Case presentation
A 29-year-old female with past medical history of hydronephrosis and insertion of left ureteral stent for nephrolithiasis presented with large bowel obstruction. Flexible sigmoidoscopy revealed a partially obstructing tumor in the sigmoid colon with adenomatous glandular epithelium and high-grade dysplasia. A 5 mm right lower lung nodule was found on computerized tomography (CT) scan of the chest, but otherwise no evidence of metastatic disease was present in scans of the abdomen or pelvis. At the time of her hospitalization, her level of carcinoembryonic antigen (CEA) was elevated at 24.1 ng/mL and both cancer antigens 19-9 and 125 were within normal limits.
She subsequently underwent upfront low anterior resection in addition to ureterolysis and left salpingo-oophorectomy due to direct extension of the tumor into the left ureter. Pathology revealed invasive adenocarcinoma of the sigmoid colon, with 3 positive lymph nodes, and negative resection margins resulting in stage pT4bN1b disease. Adjuvant 5-FU, oxaliplatin, and leucovorin (FOLFOX) chemotherapy was administered for 10 cycles. Five months postoperatively, her CEA level had decreased to 1.1 ng/mL. At this time, additional chemoradiation was recommended to improve local control and prevent recurrence. Due to fertility concerns, the gravida 2, para 1 patient underwent interval transposition of the right ovary into the right lower quadrant and right salpingectomy. Afterwards, the patient received 3-D conformal radiation therapy to the rectum with parameters of 4,500 cGy in 25 fractions with concurrent 825 mg/m2 capecitabine taken twice daily for 5 days per week.
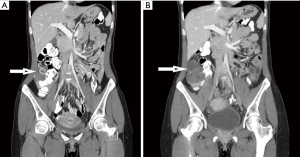
One month after completion of chemoradiation, and 10 months since initial diagnosis, the patient presented to the emergency department (ED) with abdominal pain, with CT scans showing possible ovarian torsion. Restaging CT scans completed one month after her ED stay revealed an enlarged, cystic right ovary with ovarian torsion excluded by ultrasound (Figure 1). At that time, CEA levels also began to trend upwards, reaching a peak of 68.4 ng/mL two months after completion of chemoradiation. Diagnostic laparoscopy, at this point 1 year after initial diagnosis of colon cancer, revealed right ovarian metastasis with no evidence of peritoneal metastases. Right oophorectomy successfully cleared the ovarian mass. The ovary measured 8.0×6.5×6.3 cm3 and weighed 240 grams. Tumor replaced almost the entire ovarian parenchyma and grossly appeared multicystic, with friable to solid, tan-white to tan-yellow, nodules occupying the majority of the cystic spaces. On histology, the tumor was entirely composed of large glands with garland-like pattern, extensive luminal necrosis and occasional calcifications, consistent with metastatic adenocarcinoma from colonic origin (Figure 2). A panel of immunohistochemistry further supports the diagnosis.
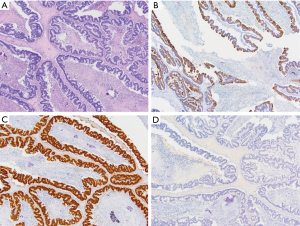
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We present a case of a 29-year-old female who underwent ovarian transposition of the right ovary as a fertility sparing measure before initiating radiation for primary left-sided colon adenocarcinoma. Months later, she was diagnosed with a right ovarian metastasis requiring right oophorectomy. For this patient, and for others facing critical decisions about ovarian preservation in advanced colorectal cancer, the question remains how to balance fertility concerns with optimal minimization of metastasis and recurrence (Table 1). There is no clear consensus on when ovarian preservation is permissible, especially in the premenopausal population.
Table 1
| Ovarian preservation | Prophylactic oophorectomy |
|---|---|
| Equal survival outcomes | Addresses microscopic disease |
| Increased overall survival has not been demonstrated in premenopausal patients with CRC who underwent prophylactic oophorectomy | If peritoneal involvement is present and one ovary presents with metastases, there is a 45% chance the remaining ovary harbors microscopic disease (6) |
| Avoids hormonal complications | Eliminates high incidence of recurrence in remaining ovary |
| Avoids low estrogen levels which are associated with cardiac, bone loss, and neurological complications; may require hormone replacement therapy (7) | If only the affected ovary is removed at initial surgery, 4 out 5 patients recurred in the remaining ovary (4) |
| Prevents sterilization | Prevents reoperation |
| Patients avoid sterilization and expensive fertility interventions (egg donors, IVF) if future pregnancies are desired | Declining status may prohibit additional operations when recurrence is discovered (8) |
| Minimizes psychological impact | Avoids involvement in new anatomic regions |
| Allows patients to focus on CRC management and associated treatment and lifestyle changes, avoids additional sense of loss | By relocating the ovary into a new anatomic location, previously uninvolved structures face heightened risk of involvement should recurrence develop in the transposed ovary |
Review of the available literature has shown metastasis to the ovary is present in 0.8–7.4% of colorectal cancer cases (2). The route of spread from the large bowel to the ovary is not definitively known, but may include transcoelomic spread, hematogenous metastasis, lymphatic spread, and direct extension (Figure 3). While our patient presented with direct tumor extension into the left ovary at initial surgery, it is less clear the mode of metastasis responsible for the metachronous metastasis to the right ovary. As summarized in Hanna and Cohen, metachronous metastasis to the ovary is uncommon, occurring in 1.4–6.8% of colorectal cancer cases, and usually occurs within 2 years of primary resection (2). Ovarian metastasis is more common in premenopausal patients, which may be associated with their stronger vascular supply, creating a “sanctuary” for metastasis.
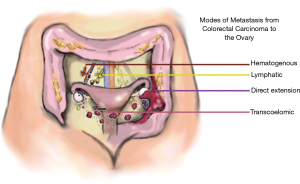
For patients like ours, who initially present with synchronous ipsilateral metastasis, the question of how to best address the contralateral ovary remains. Retrospective data in patients with CRC with peritoneal involvement suggests that if one ovary is involved, there is a 45% chance that the second ovary will have microscopic involvement (6). However, it is unclear whether this data can be extrapolated to our patient, who did not exhibit peritoneal involvement. In one study, of the five patients who presented with synchronous ovarian metastasis and underwent removal of only the affected ovary, four experienced recurrences in the remaining ovary (4). Additional case reports have pointed to the seriousness of metastasis to the remaining ovary, highlighting that the patient’s condition may not permit additional operations (8). Morbidity and mortality are greatly increased for patients with ovarian metastasis compared to those without, with a median survival of just 20 months (9). For this reason, there is considerable interest in preventing ovarian metastasis when feasible.
While initially it may seem that prophylactic bilateral oophorectomy would be advisable, there is considerably disagreement regarding its role, particularly in premenopausal patients. Notably, increased overall survival due to prophylactic bilateral oophorectomy has only been demonstrated in postmenopausal women (10,11). Proposed advantages include potential removal of microscopic synchronous metastases, reduction of metachronous metastases, and eliminating the need for repeated or emergent operation (2). No conclusions have been drawn on its benefit in premenopausal patients. However, bilateral prophylactic oophorectomy comes with risk of significant morbidity and potential development of cardiac, bone, and neurological complications if patients do not receive estrogen replacement therapy (7). Oophorectomy may also cause significant psychological stress and necessitate invasive and expensive fertility treatment.
Thus, the use of prophylactic bilateral oophorectomy in premenopausal women requires careful and individualized consideration. Facing a lack of evidence for survival benefit, hormonal complications, and sterilization, some choose not to pursue prophylactic oophorectomy. However, their fertility concerns often extend to the medical and radiation therapies used to control their primary malignancy. Radiation therapy can effectively sterilize women in their 30 s, with estimates of 14.3 Gy inducing ovarian failure and the threshold for ovarian preservation is limited at just 2 Gy (5). These patients may undergo ovarian transposition, a surgical procedure to relocate the ovary while maintaining its native blood supply, removing it from the radiation field, and preserving reproductive potential. For patients such as ours, with involvement at one ovary, and the intention of future pregnancies, the decision to undergo ovarian transposition of the remaining ovary is largely dictated by personal choice, as little data instructs an obvious clinical decision. However, our patient now faces disruptive clinical symptoms and reoperation as well as the risk of reducing survival outcomes. There is additional concern that transposition of an ovary harboring microscopic disease may effectively spread the disease to new sites in the abdomen or peritoneum.
While it is difficult to make any firm conclusions on the treatment of similar cases, it is important to note the growing body of case reports pointing to metastasis and poor outcomes in patients who chose not to undergo prophylactic bilateral oophorectomy (Table 2). Our case addresses a premenopausal patient with fertility concerns, for whom the consequences of unnecessary oophorectomy, or of undertreated malignancy, are particularly devastating. The case is unique in presenting recurrence in a transposed ovary. Providing informed, scientifically guided medical advice to such patients will depend on future studies investigating the outcomes of prophylactic surgical intervention before chemoradiation.
Table 2
| Preoperative diagnosis | Primary operation | Menstruation status | Outcome | Reference |
|---|---|---|---|---|
| Mucinous adenocarcinoma of appendix, right colon adenocarcinoma | Right hemicolectomy | Premenopausal | Left ovarian metastasis, widespread disseminated disease and death | (12) |
| Rectal carcinoma | Anterior resection | Premenopausal | Right ovarian metastasis at 16 months, left ovarian metastasis at 31 months, death at 36 months | (8) |
| Rectal carcinoma with left ovarian metastasis and peritoneal involvement | Anterior resection and left salpingo-oophorectomy | Premenopausal | Right ovarian metastasis at 16 months, death at 32 months | (8) |
| T3N1M0 colon adenocarcinoma | Right hemicolectomy | Premenopausal | Bilateral ovarian metastasis at 23 months | (13) |
| Transverse colon carcinoma | Transverse colectomy | Premenopausal | Left ovarian metastasis at 1.5 years, death at 5 years | (14) |
| Cecal adenocarcinoma with right ovarian metastasis | Right hemicolectomy and right oophorectomy | Postmenopausal | Right ovarian metastasis at 8 years | (15) |
| Carcinoma of descending colon and simultaneous hepatic metastasis | left hemicolectomy and hepatectomy | Postmenopausal | Ovarian metastasis at 1 year post chemotherapy | (16) |
| Ascending colon carcinoma | Right hemicolectomy | Postmenopausal | Right ovarian metastasis at 7 months, left ovarian metastasis at 58 months | (8) |
| T3N0M0 adenocarcinoma of sigmoid colon | Left hemicolectomy | Postmenopausal | Right ovarian metastasis at 3 years | (17) |
Case reports identified via PubMed search in August 2021, keywords “colorectal metastasis to ovary”, “recurrence ovarian transposition” and “metachronous ovarian metastasis” with case report filter, selected for relevance.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE checklist. Available at https://dx.doi.org/10.21037/jgo-21-558
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-558). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Hanna NN, Cohen AM. Ovarian neoplasms in patients with colorectal cancer: understanding the role of prophylactic oophorectomy. Clin Colorectal Cancer 2004;3:215-22. [Crossref] [PubMed]
- Herrera-Ornelas L, Mittelman A. Results of synchronous surgical removal of primary colorectal adenocarcinoma and ovarian metastases. Oncology 1984;41:96-100. [Crossref] [PubMed]
- Kammar PS, Engineer R, Patil PS, et al. Ovarian Metastases of Colorectal Origin: Treatment Patterns and Factors Affecting Outcomes. Indian J Surg Oncol 2017;8:519-26. [Crossref] [PubMed]
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys 2005;62:738-44. [Crossref] [PubMed]
- Mehta AM, Bignell MB, Alves S, et al. Risk of Ovarian Involvement in Advanced Colorectal or Appendiceal Tumors Involving the Peritoneum. Dis Colon Rectum 2017;60:691-6. [Crossref] [PubMed]
- Berek JS, Chalas E, Edelson M, et al. Prophylactic and risk-reducing bilateral salpingo-oophorectomy: recommendations based on risk of ovarian cancer. Obstet Gynecol 2010;116:733-43. [Crossref] [PubMed]
- Yamaguchi T, Takahashi H, Kagawa R, et al. The role of prophylactic bilateral oophorectomy at the time of initial diagnosis of a unilateral ovarian metastasis in cases with colorectal adenocarcinoma. Hepatogastroenterology 2008;55:434-7. [PubMed]
- Rayson D, Bouttell E, Whiston F, et al. Outcome after ovarian/adnexal metastectomy in metastatic colorectal carcinoma. J Surg Oncol 2000;75:186-92. [Crossref] [PubMed]
- Sianesi M, Bertocchi E, Rossini M, et al. Ovarian metastases from colorectal cancer: prognostic role of prophylactic oophorectomy. A single center experience. Eur J Gynaecol Oncol 2016;37:792-5. [PubMed]
- Young-Fadok TM, Wolff BG, Nivatvongs S, et al. Prophylactic oophorectomy in colorectal carcinoma: preliminary results of a randomized, prospective trial. Dis Colon Rectum 1998;41:277-83; discussion 283-5. [Crossref] [PubMed]
- Almas T, Ullah M, Kaneez M, et al. Gone but Not Forgotten: Ovarian Metastasis From a Colon Carcinoma in a 19-Year-Old Female. Cureus 2020;12:e9466. [Crossref] [PubMed]
- Sahai P, Mohanti BK, Raina PK, et al. Colon cancer with metachronous presentation of Krukenberg's tumor in an adolescent. J Gastrointest Cancer 2014;45:144-7. [Crossref] [PubMed]
- Takagi T, Nakase Y, Fukumoto K, et al. A patient with metachronous ovarian metastases of signet-ring cell carcinoma of the transverse colon showing long-term survival after surgery. Gan To Kagaku Ryoho 2009;36:2260-2. [PubMed]
- Shigeyoshi I, Komori K, Kinoshita T, et al. A case of metachronous left ovarian metastasis 8 years after surgery for cecal cancer and right ovarian metastasis: Report of a case. Nagoya J Med Sci 2017;79:259-66. [PubMed]
- Eto E, Ito Y, Mihara K, et al. A case of metachronous ovarian metastasis after curative surgery of colon cancer with simultaneous hepatic metastasis. Gan To Kagaku Ryoho 2013;40:1927-9. [PubMed]
- Paramythiotis D, Goulas P, Moysidis M, et al. Metachronous Ovarian Metastases in a Patient with Primary Colorectal Cancer. A Case Report and Review of the Literature. Am J Case Rep 2019;20:1515-20. [Crossref] [PubMed]


