
Pattern of distant metastases and predictive nomograms in colorectal mucinous adenocarcinoma: a SEER analysis
Introduction
Colorectal cancer (CRC) is a leading cause of morbidity and mortality worldwide (1). There is mounting evidence that the incidence of CRC is increasing, and approximately half of CRC patients develop distant metastasis (2). CRC classification is based on histology. Adenocarcinoma (AC) accounts for 85% of CRCs, while mucinous adenocarcinoma (MC) accounts for 10–15%. MC is characterized by abundant mucus secretion that accounts for at least 50% of tumor volume (3). Mucinous colorectal AC more frequently affects the proximal colon than the rectum or distal colon (4). It is known that relative to non-MC, MC is associated with advanced stage and distant metastases at diagnosis (5). However, the clinicopathological features and metastasis pattern of MC are controversial, which complicates treatment strategy (6-8). Thus, better understanding of the clinicopathological characteristics of MC, especially its metastasis pattern and prognostic factors, is needed.
With the exception of chemotherapy, few studies have examined the treatment of metastatic MC (mMC) with radiotherapy and surgery (9). Moreover, there are no reliable guidelines on mMC management. Thus, it is necessary to study the factors affecting MC treatment.
Based on the Surveillance, Epidemiology, and End Results (SEER) database, we systematically summarized the clinicopathological features and metastasis patterns of mMC patients. We also performed Cox regression analysis of the prognostic factors and subgroup survival analysis of these patients. We then constructed a survival prediction nomogram of the metastasis patterns. We present the following article in accordance with the TRIPOD reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-824).
Methods
Data collection
We used SEER datasets released in November 2016, which included additional treatment fields. Strict quality control is maintained by the SEER Quality Improvement Program, which establishes standards for cancer registries and maintains them through continual monitoring, assessment, and education. Since SEER is publicly available, the use of its data does not require the informed consent of patients or ethical approval.
The following criteria were used to identify eligible patients: (I) those diagnosed between 2010 and 2015; (II) primary site was colorectal MC according to the 3rd edition of the International Classification of Diseases for Oncology [ICD-O-3/World Health Organization (WHO) 2008]; (III) diagnosis confirmed by histopathology; and (IV) tumor staging performed according to the American Joint Committee on Cancer (AJCC) (7th edition). Patients lacking sufficient survival data, those whose first malignant primary tumor was not colon MC, and those with a survival of <1 month were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Participant variables and outcomes
The following clinicopathological parameters were analyzed: year of diagnosis [2010–2015], age (<65, ≥65 years), gender (female, male), race (black, white, and others), primary site (left-sided colon, right-sided colon, and unknown), grade (grade I/II, grade III/IV, and unknown), AJCC T-stage (T0–4, Tx), N-stage (N0–2, Nx), chemotherapy (no, yes), radiotherapy (no, yes), surgery (no, yes), liver metastasis (no, yes), bone metastasis (no, yes), lung metastasis (no, yes), and brain metastasis (no, yes). Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or last follow-up.
Statistical analysis
MC patients were classified by metastases site (liver, bone, lung, and brain). The Chi-square test was used to compare the clinicopathological characteristics among different metastasis sites. Survival analysis was performed using Kaplan-Meier analysis and survival differences between groups were evaluated using the log-rank test. A multivariate Cox proportional hazards model was used to determine the independent prognostic factors, and associated hazard ratios (HRs) with corresponding 95% confidence interval (CI) were generated. A nomogram based on these prognostic factors was then generated for predicting OS. Harrell’s concordance-index (C index) was used to evaluate the predictive performance of the nomogram. The nomogram was used to evaluate the consistency of prediction and observation probability. All statistical analyses were performed using R (version 4.0.4, www.r-project.org). Statistical significance was set at two-sided P<0.05.
Results
Patient characteristics
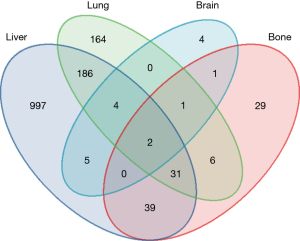
Of the 3,088 patients diagnosed with MC CRC between 2010 and 2015, 1,636 (53.0%) were <65 years old, 1,504 (48.7%) were male, and 1,584 (51.3%) were female. The primary tumor locations were as follows: 921 (39.8%) had left-side colon cancer, while 1,940 (62.8%) had right-side colon cancer. Also, 1,271 (41.2%) had initial liver metastasis, 400 (13.0%) had initial lung metastasis, 109 (3.5%) had initial bone metastasis, and 17 (0.6%) had initial brain metastasis (Figure 1).
The liver metastasis group was more likely to be >65 years old, black race, have a left side primary tumor, grade II, a lower T stage, and an advanced N stage. A lower proportion of patients with liver metastasis received surgery relative to those with non-liver metastasis. The bone metastasis group was more likely to have left side primary and lower T stage. A higher proportion of those with bone metastasis received radiotherapy relative to those without bone metastasis. Those with lung metastasis were more likely to be >65 years old, have a left side primary tumor, a lower T stage, and an advanced N stage. A higher proportion of those with lung metastasis received radiation relative to those without lung metastasis. The brain metastasis group was more likely to be Nx tumor stage. A higher proportion of those with brain metastasis received radiation relative to those without brain metastasis (Table 1).
Table 1
| Characteristic | N [%] | Liver metastasis, N [%] | Bone metastasis, N [%] | Lung metastasis, N [%] | Brain metastases, N [%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | P value* | No | Yes | P value* | No | Yes | P value* | No | Yes | P value* | |||||
| Age | <0.001 | 0.7 | <0.001 | 0.3 | ||||||||||||
| <65 years | 1,636 [53] | 1,024 [56] | 612 [48] | 1,580 [53] | 56 [51] | 1,474 [55] | 162 [40] | 1,627 [53] | 9 [53] | |||||||
| ≥65 years | 1,452 [47] | 793 [44] | 659 [52] | 1,399 [47] | 53 [49] | 1,214 [45] | 238 [60] | 1,444 [47] | 8 [47] | |||||||
| Gender | 0.068 | 0.4 | 0.7 | >0.9 | ||||||||||||
| Male | 1,504 [49] | 860 [47] | 644 [51] | 1,447 [49] | 57 [52] | 1,305 [49] | 199 [50] | 1,498 [49] | 6 [35] | |||||||
| Female | 1,584 [51] | 957 [53] | 627 [49] | 1,532 [51] | 52 [48] | 1,383 [51] | 201 [50] | 1,573 [51] | 11 [65] | |||||||
| Race | <0.001 | 0.2 | 0.3 | 0.9 | ||||||||||||
| White | 2,441 [79] | 1,456 [80] | 985 [77] | 2,361 [79] | 80 [73] | 2,135 [79] | 306 [76] | 398 [13] | 1 [6] | |||||||
| Black | 399 [13] | 199 [11] | 200 [16] | 379 [13] | 20 [18] | 337 [13] | 62 [16] | 398 [13] | 1 [6] | |||||||
| Other | 248 [8] | 162 [9] | 86 [7] | 239 [8] | 9 [8] | 216 [8] | 32 [8] | 247 [8] | 1 [6] | |||||||
| Site of the primary | <0.001 | <0.001 | <0.001 | >0.9 | ||||||||||||
| Left | 921 [30] | 455 [25] | 466 [37] | 876 [29] | 45 [41] | 747 [28] | 174 [44] | 916 [30] | 5 [29] | |||||||
| Right | 1,940 [63] | 1,236 [68] | 704 [55] | 1,890 [63] | 50 [46] | 1,762 [66] | 178 [44] | 1,929 [63] | 11 [65] | |||||||
| Large intestine NOS | 227 [7] | 126 [7] | 101 [8] | 213 [7] | 14 [13] | 179 [7] | 48 [12] | 226 [7] | 1 [6] | |||||||
| Grade | <0.001 | <0.001 | <0.001 | 0.5 | ||||||||||||
| Grade I | 390 [13] | 304 [17] | 86 [7] | 386 [13] | 4 [4] | 363 [14] | 27 [7] | 388 [13] | 2 [12] | |||||||
| Grade II | 1,252 [41] | 658 [36] | 594 [47] | 1,221 [41] | 31 [28] | 1,086 [40] | 166 [42] | 1,248 [41] | 4 [24] | |||||||
| Grade III | 506 [16] | 311 [17] | 195 [15] | 488 [16] | 18 [17] | 456 [17] | 50 [13] | 502 [16] | 4 [24] | |||||||
| Grade IV | 155 [5] | 95 [5] | 60 [5] | 153 [5] | 2 [2] | 142 [5] | 13 [3] | 154 [5] | 1 [6] | |||||||
| Unknown | 785 [25] | 449 [25] | 336 [26] | 731 [25] | 54 [50] | 641 [24] | 144 [36] | 779 [25] | 6 [35] | |||||||
| T stage | <0.001 | <0.001 | <0.001 | 0.050 | ||||||||||||
| T0 | 38 [1.2] | 23 [1.3] | 15 [1.2] | 34 [1.1] | 4 [3.7] | 28 [1.0] | 10 [2.5] | 37 [1.2] | 1 [5.9] | |||||||
| T1 | 120 [3.9] | 60 [3.3] | 60 [4.7] | 107 [3.6] | 13 [11.9] | 94 [3.5] | 26 [6.5] | 119 [3.9] | 1 [5.9] | |||||||
| T2 | 43 [1.4] | 18 [1.0] | 25 [2.0] | 41 [1.4] | 2 [1.8] | 33 [1.2] | 10 [2.5] | 42 [1.4] | 1 [5.9] | |||||||
| T3 | 744 [24.1] | 332 [18.3] | 412 [32.4] | 727 [24.4] | 17 [15.6] | 630 [23.4] | 114 [28.5] | 740 [24.1] | 4 [23.5] | |||||||
| T4 | 1,609 [52.1] | 1,087 [59.8] | 522 [41.1] | 1,575 [52.9] | 34 [31.2] | 1,473 [54.8] | 136 [34.0] | 1,604 [52.2] | 5 [29.4] | |||||||
| TX | 534 [17.3] | 297 [16.3] | 237 [18.6] | 495 [16.6] | 39 [35.8] | 430 [16.0] | 104 [26.0] | 529 [17.2] | 5 [29.4] | |||||||
| N stage | <0.001 | 0.05 | <0.001 | 0.026 | ||||||||||||
| N0 | 1,138 [37] | 809 [45] | 329 [26] | 1,106 [37] | 32 [29] | 1,022 [38] | 116 [29] | 1,132 [37] | 6 [35] | |||||||
| N1 | 783 [25] | 387 [21] | 396 [31] | 752 [25] | 31 [28] | 667 [25] | 116 [29] | 781 [25] | 2 [12] | |||||||
| N2 | 846 [27] | 429 [24] | 417 [33] | 819 [27] | 27 [25] | 735 [27] | 111 [28] | 843 [27] | 3 [18] | |||||||
| NX | 321 [10] | 192 [11] | 129 [10] | 302 [10] | 19 [17] | 264 [10] | 57 [14] | 315 [10] | 6 [35] | |||||||
| Chemotherapy | 2,034 [66] | 1,200 [66] | 834 [66] | 0.8 | 1,958 [66] | 76 [70] | 0.4 | 1,773 [66] | 261 [65] | 0.8 | 2,027 [66] | 7 [41] | 0.031 | |||
| Radiation | 150 [4.9] | 83 [4.6] | 67 [5.3] | 0.4 | 129 [4.3] | 21 [19.3] | <0.001 | 117 [4.4] | 33 [8.3] | <0.001 | 143 [4.7] | 7 [41.2] | <0.001 | |||
| Surgery | 2,240 [73] | 1,368 [75] | 872 [69] | <0.001 | 2,198 [74] | 42 [39] | <0.001 | 2,029 [75] | 211 [53] | <0.001 | 2,232 [73] | 8 [47] | <0.027 | |||
*, Pearson’s Chi-squared test.
The pattern of metastases in MC
To explore the proportions of the four main types of metastatic organs, Venn diagrams were used to illustrate the relationship between different co-metastases in mMC patients. Among the 3,088 MC patients, 1,797 patients (58.2%) had other metastasis. The liver was the only metastatic organ in 997/1,271 (78.4%) of all liver metastasis cases. The lung was the only metastatic organ in 164/400 (41.0%) of all lung metastasis cases. The bone was the only metastatic organ in 29/109 (26.6%) of all bone metastasis cases. The brain was the only metastatic organ in 4/17 (23.5%) of all brain metastasis cases. It is worth noting that the liver and lung were the most common co-metastases sites, while bone and brain were the least common co-metastases sites.
Multivariate analysis
Multivariate analysis showed that being <65 years, being non-black, grade I, N0 stage, chemotherapy, radiation, tumor surgery, and liver and bone metastasis were independent prognostic factors. Compared to those aged <65, patients aged ≥65 had worse OS (HR =1.53, 95% CI: 1.40–1.67, P<0.001). Furthermore, compared to black race patients, patients with white or other races had better OS (white race: HR =0.87, 95% CI: 0.77–0.98, P=0.023; other race: HR =0.80, 95% CI: 0.66–0.97, P=0.025). Moreover, compared to untreated patients, those treated with chemotherapy, radiotherapy, and tumor surgery had better OS (chemotherapy: HR =0.40, 95% CI: 0.37–0.44, P<0.001; radiotherapy: HR =0.58, 95% CI: 0.46–0.72, P<0.001; surgery: HR =0.36, 95% CI: 0.31–0.42, P<0.001). Compared to non-metastatic patients, those with liver and bone metastases had worse OS (liver: HR =1.46, 95% CI: 1.34–1.60, P<0.001; bone: HR =1.66, 95% CI: 1.34–2.05, P<0.001, Table 2).
Table 2
| Variables | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | |||||||
| <65 years | – | – | – | – | |||
| ≥65 years | 1.81 | 1.66, 1.97 | <0.001 | 1.53 | 1.40, 1.67 | <0.001 | |
| Gender | |||||||
| Female | – | – | – | – | |||
| Male | 1.13 | 1.04, 1.23 | 0.003 | 1.08 | 0.99, 1.17 | 0.094 | |
| Race | |||||||
| Black | – | – | – | – | |||
| Other | 0.80 | 0.66, 0.97 | 0.020 | 0.80 | 0.66, 0.97 | 0.025 | |
| White | 0.86 | 0.76, 0.97 | 0.014 | 0.87 | 0.77, 0.98 | 0.023 | |
| Site of the primary | |||||||
| Large intestine | – | – | – | – | |||
| Left | 0.63 | 0.53, 0.73 | <0.001 | 0.92 | 0.77, 1.10 | 0.300 | |
| Right | 0.46 | 0.39, 0.53 | <0.001 | 0.80 | 0.68, 0.95 | 0.011 | |
| Grade | |||||||
| Grade I | – | – | – | – | |||
| Grade II | 2.18 | 1.85, 2.57 | <0.001 | 1.73 | 1.45, 2.05 | <0.001 | |
| Grade III | 3.06 | 2.56, 3.66 | <0.001 | 2.11 | 1.75, 2.56 | <0.001 | |
| Grade IV | 2.41 | 1.90, 3.05 | <0.001 | 1.87 | 1.46, 2.40 | <0.001 | |
| Unknown | 3.45 | 2.91, 4.09 | <0.001 | 1.57, 2.31 | 1.57, 2.31 | <0.001 | |
| T stage | |||||||
| T0 | – | – | – | – | |||
| T1 | 1.01 | 0.66, 1.54 | 0.97 | 1.28 | 0.83, 1.99 | 0.300 | |
| T2 | 0.74 | 0.44, 1.25 | 0.26 | 1.25 | 0.73, 2.15 | 0.400 | |
| T3 | 0.76 | 0.52, 1.11 | 0.15 | 1.04 | 0.70, 1.57 | 0.800 | |
| T4 | 0.72 | 0.49, 1.04 | 0.082 | 1.32 | 0.89, 1.97 | 0.200 | |
| TX | 1.21 | 0.82, 1.77 | 0.34 | 1.14 | 0.77, 1.68 | 0.500 | |
| N stage | |||||||
| N0 | – | – | – | – | |||
| N1 | 1.60 | 1.43, 1.79 | <0.001 | 1.66 | 1.47, 1.87 | <0.001 | |
| N2 | 2.03 | 1.83, 2.26 | <0.001 | 2.57 | 2.26, 2.91 | <0.001 | |
| NX | 2.03 | 1.75, 2.35 | <0.001 | 1.15 | 0.98, 1.35 | 0.089 | |
| Chemotherapy | |||||||
| No | – | – | – | – | |||
| Yes | 0.48 | 0.44, 0.52 | <0.001 | 0.40 | 0.37, 0.44 | <0.001 | |
| Radiation | |||||||
| No | – | – | – | – | |||
| Yes | 0.59 | 0.47, 0.73 | <0.001 | 0.58 | 0.46, 0.72 | <0.001 | |
| Surgery | |||||||
| No | – | – | – | – | |||
| Yes | 0.43 | 0.39, 0.47 | <0.001 | 0.36 | 0.31, 0.42 | <0.001 | |
| Liver metastasis | |||||||
| No | – | – | – | – | |||
| Yes | 1.63 | 1.50, 1.78 | <0.001 | 1.46 | 1.34, 1.60 | <0.001 | |
| Bone metastasis | |||||||
| No | – | – | – | – | |||
| Yes | 2.12 | 1.73, 2.60 | <0.001 | 1.66 | 1.34, 2.05 | <0.001 | |
| Lung metastasis | |||||||
| No | – | – | – | – | |||
| Yes | 1.61 | 1.44, 1.81 | <0.001 | 1.09 | 0.96, 1.23 | 0.200 | |
| Brain metastases | |||||||
| No | – | – | – | – | |||
| Yes | 1.88 | 1.13, 3.13 | 0.015 | 1.79 | 1.06, 3.01 | 0.028 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval.
Survival outcomes
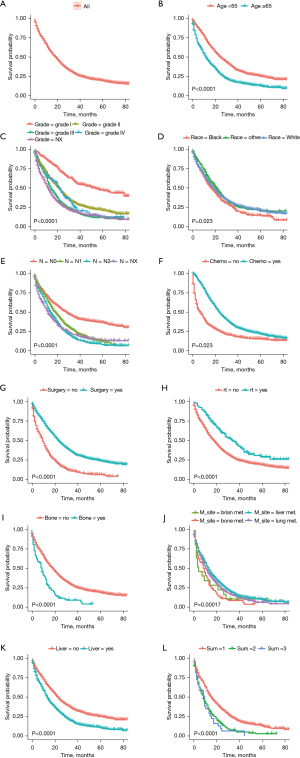
Survival analysis was performed in patients using the clinicopathological information, the treatment conducted to the primary tumor and/or metastases accordingly, as well as the metastasis sites and number of metastatic organs. We observed the following: (I) compared to those aged >65 years, patients aged <65 years had better OS (P=0.0001); (II) compared to black race patients, white and other race patients had better OS (P=0.023); (III) compared to those with advanced grade and T stage, those with lower grade and T stage had better OS (P<0.0001); and (IV) compared to untreated patients, those who underwent chemotherapy, surgery, and radiotherapy had better OS (P<0.0001).
Moreover, there were marked differences (P<0.001) between the OS of those with liver and bone metastasis compared to those without. Also, patients with liver metastasis had the best survival, while those with bone and brain metastasis had the worst survival (P<0.001). Patients with single metastasis sites had better prognosis than those with two or three metastasis sites (P<0.001, Figure 2).
Construction and validation of the nomogram in mMC
By integrating the distant metastases organs and well-known prognostic factors, a nomogram was constructed using the observed cohort to predict OS (Figure 3A). The nomogram had a c-index of 0.745 (95% CI: 0.735–0.755), indicating excellent discrimination. The calibration curve revealed good performance compared to an ideal model with regards to 12-, 18-, and 24-month OS probability (Figure 3B-3D).

Discussion
To better understand the relationship between clinicopathological features, metastasis patterns, and survival outcomes, we included a large number of patients with mMC from the SEER database. This population-based study has three main findings: (I) liver and lung are the main MC metastasis sites; (II) chemotherapy, radiotherapy, and surgery improve mMC prognosis (especially surgery); and (III) multivariate analysis revealed that being ≥65 years old, higher grade, higher N staging, and liver and bone metastasis correlate with worse median OS (mOS). Liver metastasis had better mOS relative to brain metastasis, and fewer metastatic organs had better mOS.
Numerous studies have shown that MC is more frequently associated with metastatic disease and multiple-site metastases. Our findings confirmed that MC predominantly metastasizes to the liver, followed by the lung, while the incidence of bone and brain metastases was much lower. Previous studies have also reported that the incidence of bone and brain metastases is 10–15% and 1–3%, respectively, in CRC patients (10,11). In this study, we observed a lower incidence of brain metastasis incidence (0.55%).
MC patients have been reported to have higher rates of right/transverse colon cancer (12), which is consistent with our results. Moreover, we found that right side primary MC patients had a higher incidence rate of liver, lung, bone, and brain metastases. Numerous studies have shown significant epidemiological, clinical, and histological differences in left versus right CRC (13). Comprehensive analysis of the four metastasis sites showed that metastasis was more likely to be low T stage and advanced N stage. We speculate that advanced N stage indicates an increased risk of metastasis.
We also found statistically significant differences in race distribution for liver metastases, and age distribution for liver and lung metastases. Race differences may be explained by disparities in access to health care, while the impact of age may be due to differences in baseline physical condition and comorbidities (14).
Chemotherapy is the main treatment for mMC, although surgical resection and radiotherapy are also used (9). Ott et al. proposed that combined chemotherapy protocols are effective for patients with mMC (15). In this subtype, mucus around the tumor cells may be a physical barrier to drugs. Additionally, there is a protective layer of extracellular mucin around metastatic foci in advanced MC patients. Furthermore, a large amount of mucus around the tumor causes abnormal microvascular development, which may reduce drug delivery. Moreover, compressive force (solid stress) from the giant mucinous tumor on the vascular system may further reduce drug delivery to the tumor. The development of chemotherapeutic agents that overcome these may improve MC treatment outcomes. For instance, nanoparticle drug carriers that can cross the mucosal barrier may improve the efficacy of chemotherapy against MC (16). Drugs targeting the mucus layer itself may also provide another effective choice for systemic therapy (17).
In-depth subgroup analysis of factors affecting therapy found that OS was significantly improved in patients who were recommended for chemotherapy, radiotherapy, and surgery. Multiple organ resection improves OS without increasing short-term mortality (18). Thus, appropriate surgery is critical to improving OS in mMC patients. Similarly, we found that radiotherapy, which is often used in patients with left colon cancer and rectal cancer, confers survival benefits. Our findings showed that chemotherapy, radiotherapy, and surgery are protective factors in MC.
Of these treatments, surgery may be the most important. A previous retrospective study suggested that surgical resection of primary tumors and synchronous liver metastasis was always unnecessary (19). Nevertheless, where possible, current guidelines recommend resection of both primary CRC and distant disease with curative intent in patients with liver and/or lung metastases. Radical resection of primary and distant metastases may improve long-term survival and cure rates (20).
There are several reasons that account for the survival benefits of surgical intervention for both primary and metastases sites. Firstly, resection of primary and metastases sites could reduce the tumor burden, thereby improving the response to chemotherapy. Secondly, surgical resection of primary and metastatic tumors may restore the patient’s immune function. Thirdly, surgical excision may reduce obstruction, perforation, and other complications associated with master surgery mortality and morbidity (21,22). However, careful consideration is needed before surgery in patients with multiple organ metastases. Thus, multidisciplinary team meetings may guide appropriate clinical decisions in MC patient management.
MC patients have been reported to have lower progression-free survival (PFS) and shorter mOS (15,18). However, other studies found that poor prognosis mainly existed in stage III and IV, and rectal cancer, but not colon cancer (4,23). The poor prognosis of stage IV MC may be due to its metastasis pattern. MC patients are more likely to have multiple-site metastases, and the distribution and composition of metastases differs from that of non-MC patients. In addition to liver metastasis, distant lymph node or peritoneal surface metastases are also common MC metastases (23-25). The presence of these metastases, especially in the peritoneum, is associated with very poor prognosis (26).
Our study found that patients with fewer metastatic sites had better mOS relative to those with higher numbers of organ involvement. This result is consistent with the principle of AJCC staging. On the contrary, no survival benefit was observed between patients with fewer versus additional metastatic sites in pancreatic cancer, indicating that this phenomenon is not found in all metastatic diseases (27).
Considering that MC patients have a unique metastasis pattern, independent prognostic factors were estimated and compared in detail between the subgroups. Univariate Cox regression analysis found that the factors associated with poor survival are as follows: diagnosis at ≥65 years, higher tumor grade, higher lymph node staging, as well as liver and bone metastasis. Patients with liver metastasis had the best survival, while those with bone and brain metastasis had the worst. These results are similar to SEER data on CRC (28).
In recent years, a series of studies have used the SEER database for nomogram construction and studied its prognostic value in patients with metastatic CRC (29,30). In this study, age, race, grade, N stage, chemotherapy, radiation, surgery, liver metastasis and bone metastasis were used for nomogram construction. Our prognostic nomogram exhibited good prediction for OS using the SEER database (c-index: 0.745). Recent years, a series of clinical studies have provided new evidences for the treatment and management of MC patients. However, the exact molecular mechanisms of promoting MC metastasis are still unclear, and it will possibly enable further tailoring of treatment.
Although SEER datasets have high integrity and validity, our research had some limitations. Firstly, the SEER data did not provide the specific numbers of metastases. Secondly, the sequence of chemotherapy, radiotherapy, and surgery, as well as detailed information on the drug regimen and whether the patients received adjuvant or neoadjuvant therapy were not clear. Thirdly, the gene expression characteristics of the patients were not available. Thus, studies with larger sample sizes, especially randomized controlled trials, are needed to verify our findings.
Conclusions
In summary, we observed that patients with mMC had a characteristic distant metastasis pattern. We constructed a new and accurate mMC prognostic model based on population-based data. These findings can be utilized to predict prognosis and guide mCRC MC patient management.
Acknowledgments
Funding: This work was supported by the Science and Technology Project Foundation of Suzhou (No. SS201852, SS202093, SYSD2020061); the Science and Education for Health Foundation of Suzhou for Youth (No. KJXW2019074); Youth Medical Science and Technology Innovation Project of Xuzhou Health Committee (No. XWKYHT20200024).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-824
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-824). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study is a retrospective cohort study based on data from the SEER database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).The SEER Program collects data from population-based cancer registries with anonymous information. The SEER is a publicly available database; thus, no ethical approval was required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Kong J, Li Y, Bian YJ, et al. Chemoembolization with CalliSpheres drug-eluting beads loaded with irinotecan in the treatment of unresectable colorectal cancer liver metastases: preliminary results in 16 cases. Transl Cancer Res 2019;8:856-66. [Crossref]
- Luo C, Cen S, Ding G, et al. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond) 2019;39:13. [Crossref] [PubMed]
- Hugen N, Verhoeven RH, Radema SA, et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol 2013;24:2819-24. [Crossref] [PubMed]
- McCawley N, Clancy C, O'Neill BD, et al. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis Colon Rectum 2016;59:1200-8. [Crossref] [PubMed]
- Bagante F, Spolverato G, Beal E, et al. Impact of histological subtype on the prognosis of patients undergoing surgery for colon cancer. J Surg Oncol 2018;117:1355-63. [Crossref] [PubMed]
- Díaz-Vico T, Fernández-Martínez D, García-Gutiérrez C, et al. Mucinous adenocarcinoma arising from chronic perianal fistula—a multidisciplinary approach. J Gastrointest Oncol 2019;10:589-96. [Crossref] [PubMed]
- Hosseini S, Bananzadeh AM, Salek R, et al. Prognostic Significance of Mucinous Histologic Subtype on Oncologic Outcomes in Patients With Colorectal Cancer. Ann Coloproctol 2017;33:57-63. [Crossref] [PubMed]
- Mekenkamp LJ, Heesterbeek KJ, Koopman M, et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer 2012;48:501-9. [Crossref] [PubMed]
- Kawamura H, Yamaguchi T, Yano Y, et al. Characteristics and Prognostic Factors of Bone Metastasis in Patients With Colorectal Cancer. Dis Colon Rectum 2018;61:673-8. [Crossref] [PubMed]
- Sundermeyer ML, Meropol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005;5:108-13. [Crossref] [PubMed]
- Viganò L, Russolillo N, Ferrero A, et al. Resection of liver metastases from colorectal mucinous adenocarcinoma: is this a different disease? Results of a case-control study. Ann Surg 2014;260:878-84; discussion 884-5. [Crossref] [PubMed]
- Lee MS, Menter DG, Kopetz S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J Natl Compr Canc Netw 2017;15:411-9. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Ott C, Gerken M, Hirsch D, et al. Advanced Mucinous Colorectal Cancer: Epidemiology, Prognosis and Efficacy of Chemotherapeutic Treatment. Digestion 2018;98:143-52. [Crossref] [PubMed]
- Hugen N, Brown G, Glynne-Jones R, et al. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol 2016;13:361-9. [Crossref] [PubMed]
- Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009;9:874-85. [Crossref] [PubMed]
- Catalano V, Loupakis F, Graziano F, et al. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol 2012;23:135-41. [Crossref] [PubMed]
- Schmidt C. Metastatic colorectal cancer: is surgery necessary? J Natl Cancer Inst 2009;101:1113-5. [Crossref] [PubMed]
- Boudjema K, Locher C, Sabbagh C, et al. Simultaneous Versus Delayed Resection for Initially Resectable Synchronous Colorectal Cancer Liver Metastases: A Prospective, Open-label, Randomized, Controlled Trial. Ann Surg 2021;273:49-56. [Crossref] [PubMed]
- Page AJ, Ejaz A, Spolverato G, et al. Enhanced recovery after surgery protocols for open hepatectomy--physiology, immunomodulation, and implementation. J Gastrointest Surg 2015;19:387-99. [Crossref] [PubMed]
- Buchs NC, Ris F, Majno PE, et al. Rectal outcomes after a liver-first treatment of patients with stage IV rectal cancer. Ann Surg Oncol 2015;22:931-7. [Crossref] [PubMed]
- Numata M, Shiozawa M, Watanabe T, et al. The clinicopathological features of colorectal mucinous adenocarcinoma and a therapeutic strategy for the disease. World J Surg Oncol 2012;10:109. [Crossref] [PubMed]
- Catalano V, Loupakis F, Graziano F, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer 2009;100:881-7. [Crossref] [PubMed]
- van Gestel YR, Thomassen I, Lemmens VE, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol 2014;40:963-9. [Crossref] [PubMed]
- Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 2012;30:263-7. [Crossref] [PubMed]
- Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J Gastroenterol 2017;23:1872-80. [Crossref] [PubMed]
- Luo D, Liu Q, Yu W, et al. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: a population-based study. Int J Colorectal Dis 2018;33:1241-9. [Crossref] [PubMed]
- Tang M, Wang H, Cao Y, et al. Nomogram for predicting occurrence and prognosis of liver metastasis in colorectal cancer: a population-based study. Int J Colorectal Dis 2021;36:271-82. [Crossref] [PubMed]
- Liu Z, Xu Y, Xu G, et al. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol 2021;21:103. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


